About our project
The problem we solve: For patients with diabetes, ignorance of foot health status can be devastating medically, emotionally and financially. Diabetic patient management of blood sugar is complicated, onerous, and painful, thus leading to poor treatment compliance and increased susceptibility to secondary complications. Two of the major complications are injuries of the extremities: foot ulcerations and a bone/tissue deformity called Charcot neuropathic osteoarthropathy (Charcot). During the first phase of product testing for neuropathy patients, our smart insoles that work seamlessly with the mobile app would constantly monitor temperature and pressure of the footbed to provide the full historical data for the physician to review. Our app, in real-time, keeps patients notified if the system detects the temperature and pressure discrepancy between the left and right foot, which was clinically and scientifically proven to be one of the early symptoms for foot ulcers.
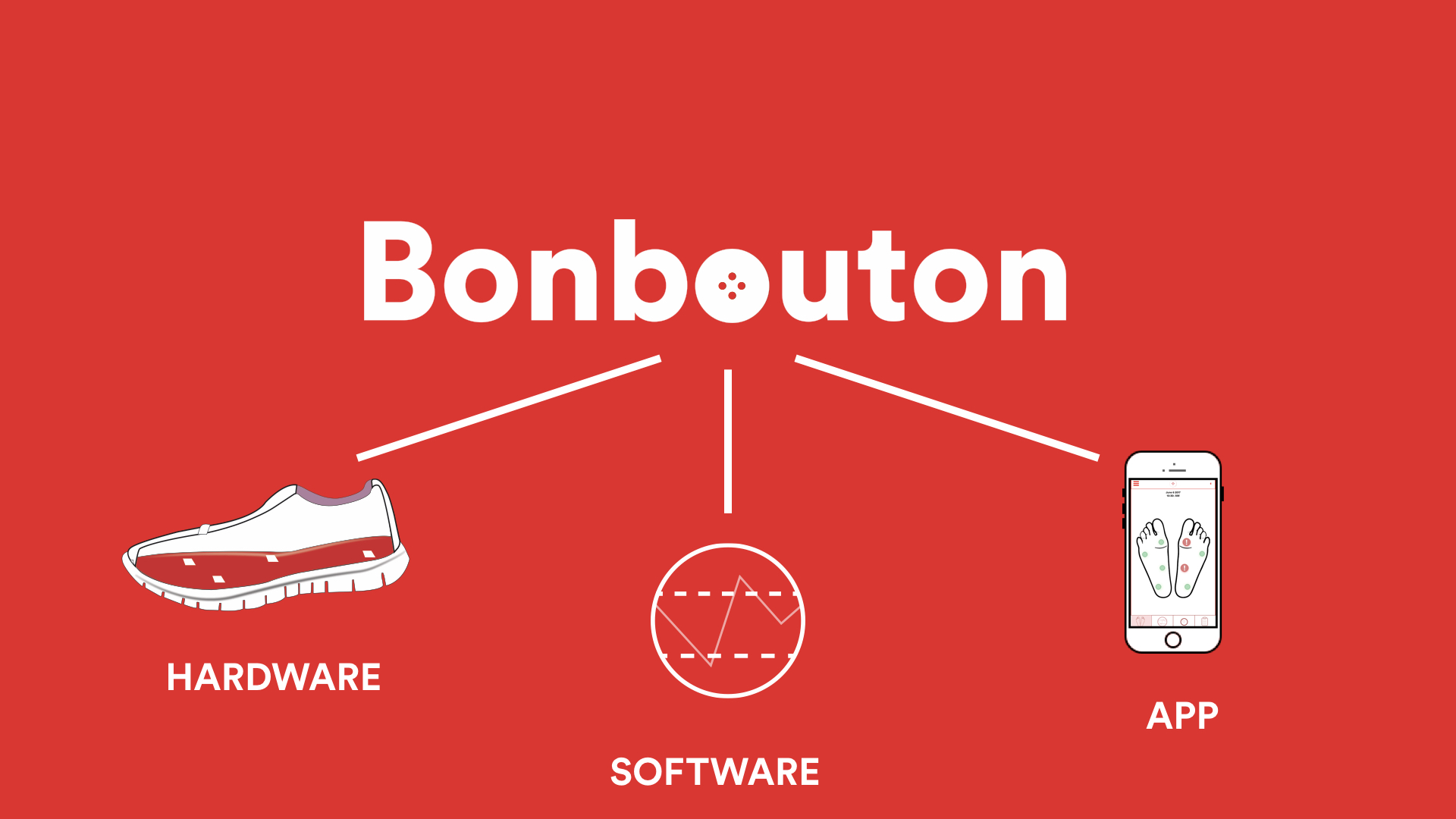
About our solution:
The suite of solutions that we're offering includes connected insoles as a physical product, and the remote monitoring dashboard for physicians to take care of patient's health via insurance reimbursement. We are in the first pilot testing phase with diabetic patients for usability studies, as well as integrating the insights about physician workflow. We further hypothesize that the implementation of our early diagnostic tool would significantly reduce the need for foot amputations and also the 5 year mortality rate in the diabetic patient group. We plan to initially partner with NYC-area podiatrist offices and orthopedic shoe to take advantage of their sale and distribution system starting in mid-2019 and expland more widely outside of New York area in the year of 2019.
Furthermore, we plan to offer our diagnostic insoles paired with Quikiks Hands-Free footwear to increase compliance and thereby efficacy of our system.
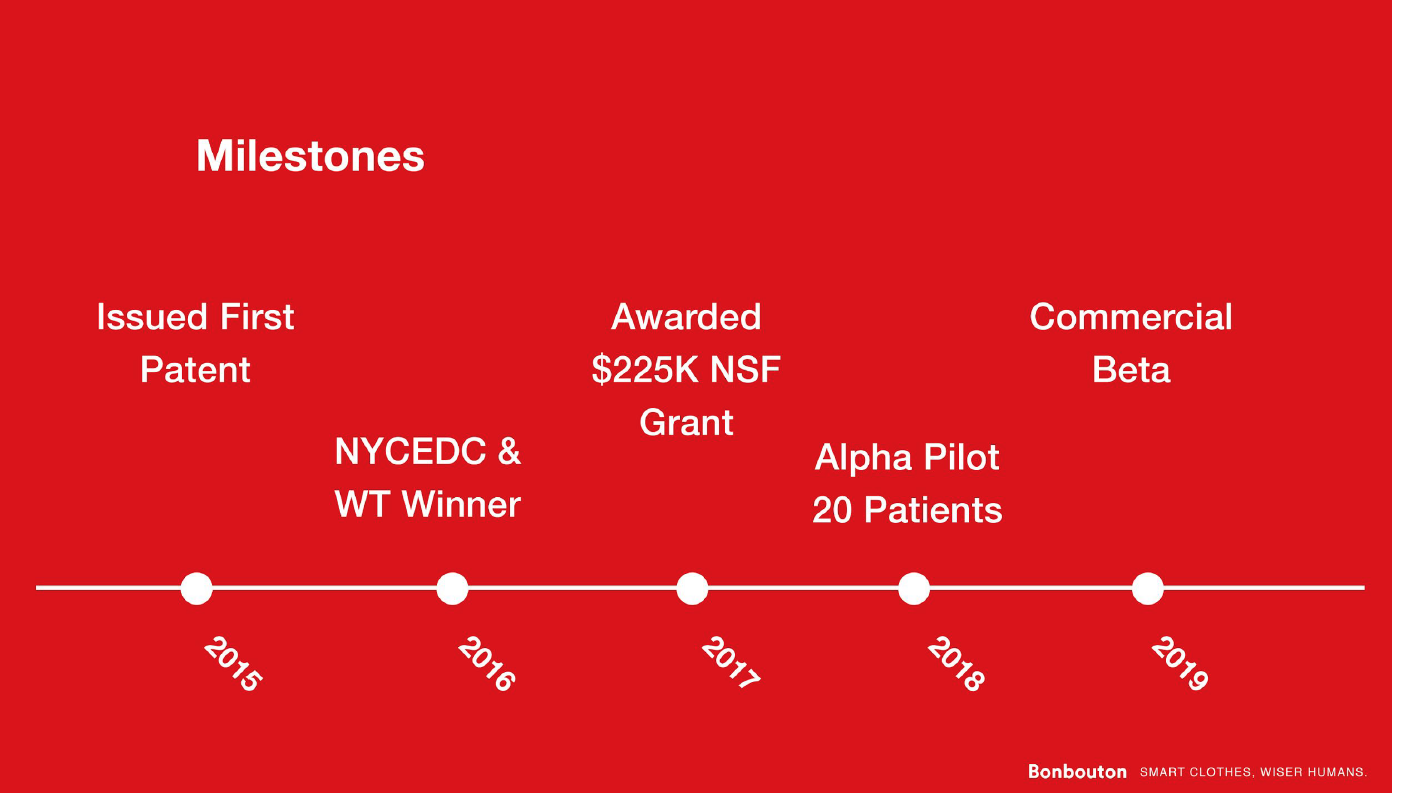 Progress to
date:
Progress to
date:The core technology was developed in 2012 during the founder’s PhD studies and there are 5 issued and 1 pending patents that cover the work. The first proof of concept device was developed in 2016 when the company received an SBIR grant from the NSF. The product was extensively tested in-house for mechanical durability and overall performance before being offered to the first customer validation in the summer of 2017. Bonbouton participated in the Digital Health Breakthrough Network, an NYCEDC-funded program, and received the first IRB to test out the product on patients. At the same time, Bonbouton received its seed funding, joining a premier accelerator, ERA in New York, followed by Brinc IoT in Hong Kong, to further explore and validate the business thesis. We are currently in contact with the Center for Medicare and Medicare Service to obtain the reimbursement procedure for diabetic patients.
The major challenges for Bonbouton lie in regulatory approval and partnerships with insurance companies. We plan to submit a 510k waiver for a class 1 device in the first quarter of 2018, while continuing with the Beta pilot for clinical study and offer the first batch of manufacturing. Once the clinical data has proven to be better than the current standard-of-care, we plan to partner with insurance companies to help reduce their cost of treating diabetic foot ulcers and amputations.
About Our Team

Creator: Linh Le
Location: New York
Bio: Linh Le is a chemical engineer by training, he has published multiple papers, international conference proceedings and currently holds six US patent. He later found Bonbouton, a technology platform for preventative diabetic healthcare. Prior to that, he received his MS in Chemical Engineering from Columbia University and BS in Chemistry from Vietnam National University. As a vivid distance runner, he recently acquired ultra-marathoner title, and had plan to run all the World Marathon Majors.
Title: Founder and CEO
Advanced Degree(s): MSc
About Team Members
Malcolm Nason
VP of R&D, Bonbouton, PhD, MBA
Biography: * MBA/PhD with over 15 years of experience in marketing, entrepreneurship, academic, industrial safety and biotech R&D including strategic development of product pipelines with an understanding of key value drivers.
* Outstanding customer development expertise with active listening skills and creative market research ability.
* Operational experience with the ability to coach and motivate individuals, empower teams, and monitor and measure performance.
* Enthusiastic communicator among varied stakeholders with the aptitude to influence project success with clearly defined cross-functional goals and motivational support.
Title: VP of R&D, Bonbouton
Advanced Degree(s): PhD, MBA
LinkedIn:
https://www.linkedin.com/in/malcolmnasonphd/
Steve Kaufman
Founder, Hands-Free LLC,
Biography: An Engineer and serial entrepreneur, Steve founded Hands-Free LLC, which manufacturers and distributes the first fully supportive, totally hands-free operable line of footwear. Its Quikiks Hands-Free Shoes, with its patented Step-in-Go Technology, allow people with physical or cognitive challenges to step easily into the shoe and in the same motion effortlessly and securely fasten their foot comfortably in place without bending over or using their hands.
Title: Founder, Hands-Free LLC
LinkedIn:
https://www.linkedin.com/in/steve-kaufman-ba0344/
About Our Company

Bonbouton
Location: 29-10 Thomson Avenue
Fl 7th, St 10
Long Island City, NY 11101
Founded: 2014
Website: http://www.bonbouton.co
Twitter: bonbouton
Product
Stage: Prototype/MVP
YTD Sales: Working on it
Employees: 3-5
How We Help Patients
We provide a novel and desired means of communication regarding the foot health of patients to patients in real-time and between patients and providers. Diabetic patients indicate that they have a great interest in foot health technology and are willing to adopt smart shoes as a prevention measure. Our solution constantly monitors the foot condition and provides the risk level for the patient directly on the smartphone app. If the foot condition exceeds the "threshold" for a healthy foot, a notification will be sent through text message to both patient and provider for a proper intervention.
How We Help Physicians
Our current customer interview with different groups of podiatrist has confirmed inial value proposition of using our solution. If we can catch the ulcer development early, Bonbouton system can potentially reduce time-consuming emergency procedure. It has been proven that such non-scheduled surgery is costly not only for the podiatrist but also the hospital.
In addition, foot sensor data can help podiatrists identify trends before patients appointment or as a remote monitoring follow up. Currently, podiatrist can get reimbursement via a CPT code for a subset of patients who is suffering from multiple chronic conditions.
How We Help Hospitals
The hospital has expressed some initial interest in reducing the operating cost by minimizing the number of amputation surgery. We further hypothesized that our solution will reduce the readmission rate after surgery, as well as improve the patient engagement experience.
How We Help Partners
We are looking for investor to join our current funding round. Please reach out if you're interested
Challenge Mission
Market Size
Our target market is people with Diabetes who have Diabetic Neuropathy and are prone to foot ulcers. There are approximately 10 million people living with this condition in the US, which represents about 30% of the whole Diabetic population. Diabetic foot amputation affects 70,000 patients a year –one every 7.5 minutes!, cost the healthcare system 15 billion dollars and have a tremendous social impact. Bonbouton’s initial addressable market, even as a fraction of $15B, is estimated at well over $100 million.
Projected 3 Year Growth
We plan to offer the first commercial pilot in 2019 and expand to the Southern states such as Florida, Georgia and Texas which are severely affected by diabetes. If we can offer our solution to 10% of the whole diabetic patients population, this 3 million pairs of shoes will produce $100M revenue by the end of 2021.
How We Will Make Money
Direct sale for diabetic insole: through reimbursement up to $300/patient/year
SaaS platform: remote monitoring: up to $200/patient/year
About our Competition
Podimetrics: FDA approved scale used by podiatrist office
Temp Touch: FDA approved thermometer used by care giver
Siren Care: Pre-production disposible socks used by diabetic patients
Progress with Customers to date
We received some early traction from both physician and patients
New Orleans and Our Company
We're looking into expanding our network. New Orleans would be a perfect place to do a pilot study.
Innovation Details
Intellectual Property Summary
The core technology of the inkjet printing of graphene sensors was developed during Linh's PhD at Stevens Institute of Technology. This technology was co-developed with the U.S. Army and would enable the fabrication of next-generation of sensors utilizing graphene nanomaterial in the most economical way. There are 5 issued patents and 1 pending as of January 2018 to protect the technology
Patent Link
http://www.freepatentsonline.com/8810996.html http://www.freepatentsonline.com/9178129.html http://www.freepatentsonline.com/9399580.html
Clinical Information
Skin temperature monitoring has been scientifically and clinically proven to reduce the risk for diabetic foot ulceration, especially in high-risk patient populations. Dr. Amstrong and Dr. Lavery, among the pioneers in the field, published multiple studies ranging from the American Journal of medicine to Diabetic Care and proved that temperature monitoring is an effective self-assessment tool for this patient population. We have received our private IRB to work with the first group of diabetic neuropathy patients to conduct the pilot studies.
Regulatory Status
We plan to submit the 510k waiver for the non-instrusive device and we have identified several predicates including a TempTouch thermometer.
How we will use the funds raised
We're raising our pre-seed round to finish the prototyping and inventory for the clinical pilot.
Thank You
The whole team is strongly motivated toward the betterment of diabetic healthcare. Linh and Malcolm have been experiencing the effect of diabetes on different family members. Steve's inspiration to invent Hand-Free shoes came from his son, Alex, who had to wear a torso brace 22 hours a day for the treatment of his scoliosis, but prevented him from independently putting his shoes on.



 Progress to
date:
Progress to
date:














