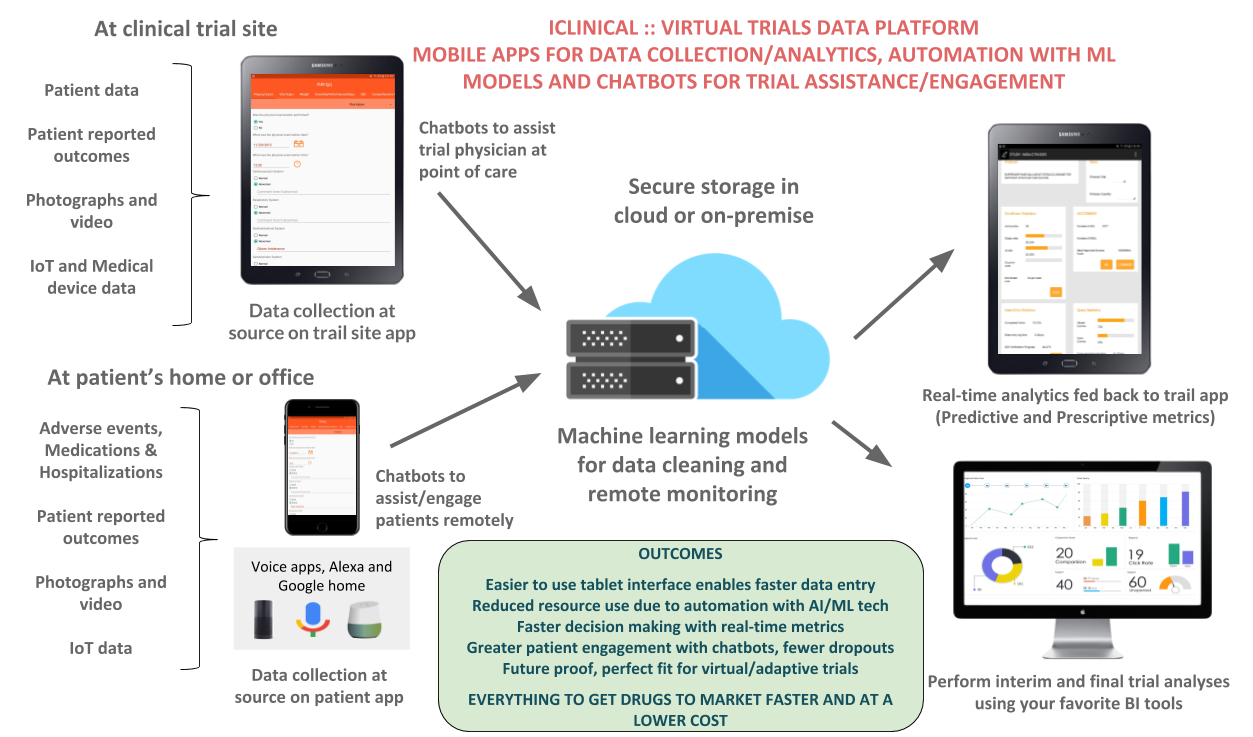
About our project
The problem we solve: Currently data collection happens on paper/EMR records. This is then transcribed into pharma portals (Medidata Rave or Oracle inform). It is stored in siloed servers. Periodic analytics is done to monitor the status of the drug trial. AI/ML technologies are not used in drug trials, automation with these technologies can reduce costs and speed up drug development.

About our solution:
Data collection happens in front of the subject (eSource). Any patient reported outcomes, video, audio and IoT/device data is again captured at source. Secure storage is in HIPAA compliant cloud servers. Real-time AI powered analytics is fed back to the study tablets. There is complete 21CFR Part 11 and HIPAA compliance, including audit trail of all the data entered, investigator signatures and secure access to the study tablets. The AI based trial assistant (chatbot) provides assistance to both patients and the trial staff. Predictive analytics provides Pharma’s with the trajectory of the trial in real-time. This can include the endpoints, site status and study outcomes.
Progress to
date:We have developed the product including the data collection and analytics. We are currently working on machine learning models and the patient app. We are starting work on three expanded access clinical trials (~100K revenue potential). We are in discussions with big pharma (Genentech, Merck, Novartis and Pfizer, CROs (DCRI and IQVIA), SMOs (Elligo Research) and Medical device companies (Abbott and Medtronic). Our go to market customers will be biotechs, small pharma and med device companies.
About Our Team

Creator: Sridhar Byrappa
Location: California
Education: Rush University, University of Illinois at Ch
Bio: Sridhar Byrappa (CEO) has 10+ years experience working on large global trials at Merck, Accenture and Wyeth. He has a PhD and an MBA.
Title: CEO
Advanced Degree(s): PhD, MBA
About Team Members
Varsha Venkatesh
CTO, Master of Science, Bachelors of Engineering
Biography: Varsha Venkatesh, our CTO is a data scientist with an MS in Computational Neuroscience from Max Planck Research Institute. She has experience working in Artificial intelligence/machine learning models both in academia and industry.
Title: CTO
Advanced Degree(s): Master of Science, Bachelors of Engineering
LinkedIn:
https://www.linkedin.com/in/varsha-v-51308398
John Stevens
CMO, Bachelors in Communication
Biography: John Stevens, our CMO, has done his bachelors in Communications and has 15+ years work experience in marketing and clinical research.
Title: CMO
Advanced Degree(s): Bachelors in Communication
LinkedIn:
https://www.linkedin.com/in/john-stevens-a06bab5/
How We Help Patients
We make participation in clinical trials easier for patients:
- With remote data collection tools patients can provide trial data remotely, which means fewer vists to clinical tiral sites.
- Patients are kept informed and engaged in the clinical trials. Our chatbots provide answers to trial specific questions.
- Finally our platform allows patients to reach the trial sponsor or monitor easily, getting their concerrns addressed quickly and remotely.
How We Help Physicians
For the trial physicians
- We provide a data platform enables trial physicians to enter trial data painlessly. This means faster trial data collection at source.
- With trial chatbots, we provide protocol related information at the point of care, this means no rummaging through documents to look for specific trial information. This chatbots can provide inclusion/exclusion criteria, trial checklists and videos of any trial procedures.
- Finally, with fewer data entry and query resolution burdens, trial investigators can enroll greater number of patients in clinical trials.
How We Help Hospitals
For hospitals, we make it painless to administer drug trials. Our mobile data platform makes it easy for hospitals to run drug trials. It drastically reduces paper work and administrative burden.
Innovation Details
Intellectual Property Summary
Our technology and process for running drug trials are unique. We customize our data platform for the clinical trial We also customize analytics and other parts of the trial. This bespoke model cannot be easily replicated by our competitors. Finally, our machine learning models for data cleaning and trial monitoring are unique and will get better over time, this will be our main IP going forward.
Clinical Information
Outcomes from our drug development model
- Easier to use tablet interface enables faster data entry
- Reduced resource use due to automation with AI/ML tech
- Faster decision making with real-time metrics
- Greater patient engagement with chatbots, fewer dropouts
- Future proof, perfect fit for virtual/adaptive trials
EVERYTHING TO GET DRUGS TO MARKET FASTER AND AT A LOWER COST
Regulatory Status
Our data platform does not need FDA approval. Our data platform has to adhere to FDA 21 CFR Part 11 guidelines, which it does. The guidelines involve having secure logins for all the trial stakeholders, complete audit trail of for all the data being entered/changed in the system and finally making sure that the prinicipal investigator signs off on all the patient data being collected in the system.
How we will use the funds raised
We will use our funds to get the data platform audited by a third party firm and we it will also be used to start 1-2 clinical trials.
Thank You
We ask you you support the project so that we can lower the cost of drug development, get greater number of life saving drugs to the market sooner and at a lower cost.