CEMENTIC: CEMENTIC
Lipid Nanoparticles to improve root canals and beyond
Palo Alto, CA United States Medical Device Biotech Dental Equity RaiseAbout our project
The problem we solve: Root canal treatments fail in 50% of cases, leading to persistent infections, antibiotic overuse, pain, repeated procedures, and increased healthcare costs.

About our solution: Cementic developed EndoNu™, an innovative nano-bioceramic root canal sealer that eliminates 99.9% of bacteria, actively rebuilds dentine, and releases antiseptics continuously, reducing root canal failures by half.
Progress to date:
Cementic has developed a pilot batch ( final product at scale), EndoNu™, validated through extensive preclinical studies demonstrating its safety and superior antimicrobial efficacy. We completed successful biocompatibility and ISO 10993 evaluations, preparing for regulatory submissions. Strategic partnerships are established with leading dental distributors including Henry Schein, and collaborations are underway with key dental manufacturers like FKG and Produits Dentaires. The dental and scientific community has strongly welcomed our innovation, highlighted by positive feedback, interest from industry experts, and publications in top dental journals.
About Our Team

Creator: Samir Raddi,DDS, Ph.D
Location: California
Education: Universite of Paris Descartes, NYU , HEC Bus
Bio: Samir Raddi, DDS, Ph.D., is a French dentist-scientist and bio-entrepreneur recognized as co-founder and CEO of CEMENTIC, a deep-tech startup creating antiseptic bioceramics designed to significantly reduce root canal failures. Balancing clinical practice and innovation, he splits his time between his private practice in Paris’s 16?? arrondissement and an R&D lab at Stanford’s StartX accelerator in Palo Alto. Raddi pursued an interdisciplinary education, earning an M.Sc. in Genetics from Université Paris Descartes (2013), an M.Sc. in Tissue & Cell Biotherapies from Arts et Métiers ParisTech (2015), and his DDS from Université Paris-Descartes (2017). He completed advanced training in Periodontology & Implantology at NYU (2021) and a Ph.D. in Sciences at Université Claude-Bernard Lyon 1 (2024). His clinical training spans roles at Hôpital Bretonneau, Paris, and the University of Iowa Hospitals and Clinics, USA. While at Lyon, his research shifted toward developing controlled-release nanomaterials for endodontics, notably publishing pioneering findings in the Journal of Endodontics on chlorhexidine-loaded lipid nanoparticles embedded in bioceramic sealers. In 2018, Raddi founded CEMENTIC, directing an Anglo-French team across Paris and Silicon Valley. Their flagship product, EndoNu™, incorporates antiseptic liposomes within a calcium-silicate matrix, undergoing preclinical evaluations at StartX facilities and Paris Saclay Raddi holds multiple patents, prominently US 2020/0315923 A1, covering disinfectant bone and dental filler materials using liposomes. His startup was notably spotlighted at Paris-Saclay SPRING 50 (2025) and leverages Stanford’s extensive entrepreneurial ecosystem. Actively engaged clinically, Raddi maintains weekend clinics specializing in implants, bone grafting, alongside. He exemplifies clinician-led innovation driving advances in global oral healthcare.
Title: CEO & Co-Founder
Advanced Degree(s): DDS,PhD
About Team Members
Badiaa El Karmy
CMO, MD, MSc
Biography: Badiaa El Karmy, MD, MSc, is the Chief Medical Officer at CEMENTIC, a dual-hub deep-tech med-biotech company based in Paris and Palo Alto. With a robust clinical and translational background, she is essential in aligning CEMENTIC’s antiseptic bioceramic technology with clinical needs and regulatory requirements.
Before joining CEMENTIC, Dr. El Karmy earned her MD from Geneva University and a Master’s degree in Medical Statistics from Université Paris-Saclay. Her professional journey into endodontics and dental biomaterials is marked by significant roles within CEMENTIC.
At CEMENTIC, Dr. El Karmy has been influential since 2023–24, initially as Clinical Affairs Director before advancing to CMO. She oversees medical and clinical strategy, including study design, data analysis, and validation of the flagship product EndoNu™, an antiseptic-releasing bioceramic sealer. EndoNu™ uniquely integrates liposomal antiseptic within a calcium-silicate matrix and is undergoing extensive preclinical evaluations and initial clinical usage.
Dr. El Karmy has represented CEMENTIC at various prestigious events, including the AAE24 conference in Los Angeles (2024) and JPMorgan 2025 biotech meetings. Her efforts were notably acknowledged by peers, including Stephen Thaddeus Connelly from UCSF and the BioLabs Europe community, for exceptional coordination and clinical insight.
Title: CMO
Advanced Degree(s): MD, MSc
About Our Company

CEMENTIC
Location: 2627 Hanover St
Palo Alto, CA 94304
US
Founded: 2022
Website: https://cementic.com
Product Stage: Ready
YTD Sales: Working on it
Employees: 1-2
How We Help Patients
Cementic's innovation directly improves patients' long-term health by significantly reducing root canal failures—a common source of chronic infections and antibiotic overuse. By using advanced bioceramic technology with targeted antiseptic release, our solution eliminates harmful bacteria, promotes natural healing, and helps patients avoid painful, costly retreatments or implants. With healthier teeth and reduced infections, patients can maintain overall better systemic health and quality of life.
How We Help Physicians
Cementic’s innovation addresses a key frustration for dentists: repeated root canal failures negatively impact patient trust, satisfaction, and practice reputation. By leveraging nano-bioceramic technology and sustained antiseptic delivery, Cementic’s EndoNu™ significantly reduces reinfection and retreatment rates, ensuring predictable clinical outcomes. This allows dentists to provide reliable, high-quality care, enhancing their professional reputation, patient satisfaction, and practice growth.
How We Help Hospitals
Each year $40B is spent on root canals in the USA. Cementic’s EndoNu™ benefits hospitals and dental institutions by significantly decreasing the rate of root canal failures, a costly and resource-intensive challenge. Failed treatments often lead to complications requiring additional clinical interventions, antibiotic treatments, and complex implant surgeries. Hospitals can thus improve patient outcomes, reduce antibiotic usage, optimize treatment efficiency, lower procedural costs, and enhance their institutional reputation for excellence and innovation.
How We Help Partners
Cementic helps partners solve key business challenges: dental suppliers and distributors face increasing pressure to deliver innovative solutions in a highly competitive market ( from abroad). By partnering with Cementic, they gain exclusive access to advanced, evidence-based technology, which increases their market differentiation, customer loyalty, and profitability. Hospitals and clinics benefit from improved operational efficiency, fewer repeat procedures, and enhanced patient satisfaction—addressing core institutional pain points.
Innovation Details
Intellectual Property Summary
Write and filed the core patent in 2017 : Protect LNPs formulation + antiseptic+ Root canal sealer
Write and filed a new patent in 2024 : Protect Nano powder with nano-Hydryapatite ( Nasa Compound)
Patent Link
https://uspto.report/patent/app/20200315923
Clinical Information
We do not yet have clinical data for Cementic's technology; however, our extensive preclinical studies provide strong evidence of efficacy. Specifically, our ex vivo studies using extracted human teeth have demonstrated impressive antimicrobial performance, with significant bacterial load reduction within root canals. Importantly, the conditions in ex vivo root canal studies closely approximate those found clinically, as treated teeth lack active blood circulation and immune response, making our results highly indicative of real-world effectiveness.
Moreover, existing literature from King's College London supports the principle that reducing bacterial load significantly enhances clinical success (Zahran et al., King's College London, 2021).
We are currently in the regulatory preclinical stage, preparing for FDA submission under the 510(k) pathway, which does not require a clinical study (strong predicate). However a clincal study is planned in Europe to get the CE mark and to prove the superiority of our product. Any input on structuring and getting data from US patients in 2026 to avoid a clinical study in Europe will be helpful.
Regulatory Status
Biocompatibility testing made by Eurofins ( last result for September 25)
ISO 13485 certification scheduled for August 25.
USA ( funded) : 510 K , reviewed with MCRA consulting ( Former FDA director in dental department) .
CE mark ( not funded): After a 1-year clinical study to demonstrate the safety and superiority of our product => to get health insurance reimbursement.
How we will use the funds raised
- Operational cost: Produce 1 GMP batch and 1 commercial batch. Commercialization in the USA includes 2 additional commercial batches (we have an LOI from Henry Schein).
- R&D: Internalize part of the production to manage COGS and conduct the clinical study
- Team: RA/QA director, business developer, production engineer, R&D assistant.
- Operational cost: Production of 1 GMP batch and 1 commercial batch, Commercialization in the USA with 2 additional commercial batch ( we have an LOI from Henry Shein)
- R&D : Internalize part of the production to manage the COGS, Clinical Study,
- Team : RA/QA director , Business Developer, Production engineer , R&D assistant
Thank You
Root canal failure causes real pain, infections, and unnecessary antibiotic use and implants for millions. We're dedicated to making that happen by applying biotech and nanotech to dramatically improve patient care. Your support and vote on MedStartr will help us transform dentistry for patients and dentists alike. Together, we can make a meaningful difference and end a public health issue forever.
Investor Info
Market Size
The global endodontics market (root canal therapy and related products) is large andsteadily growing. In 2025, the market for root canal treatment materials is estimated at ~$2.5billion globally and is projected to reach ~$3.25 billion by 2030 (4.8% CAGR). Within this,Cementic’s initial focus is on the consumable root canal sealer segment in key regions(North America and Europe), roughly a $350 million/year serviceable market. The root canal market is one of the fastest-growing segments, projected to reach $1.2 billion by 2030. Thissegment encompasses over 80 million procedures per year that could directly benefit fromEndoNu™’s superior infection control.
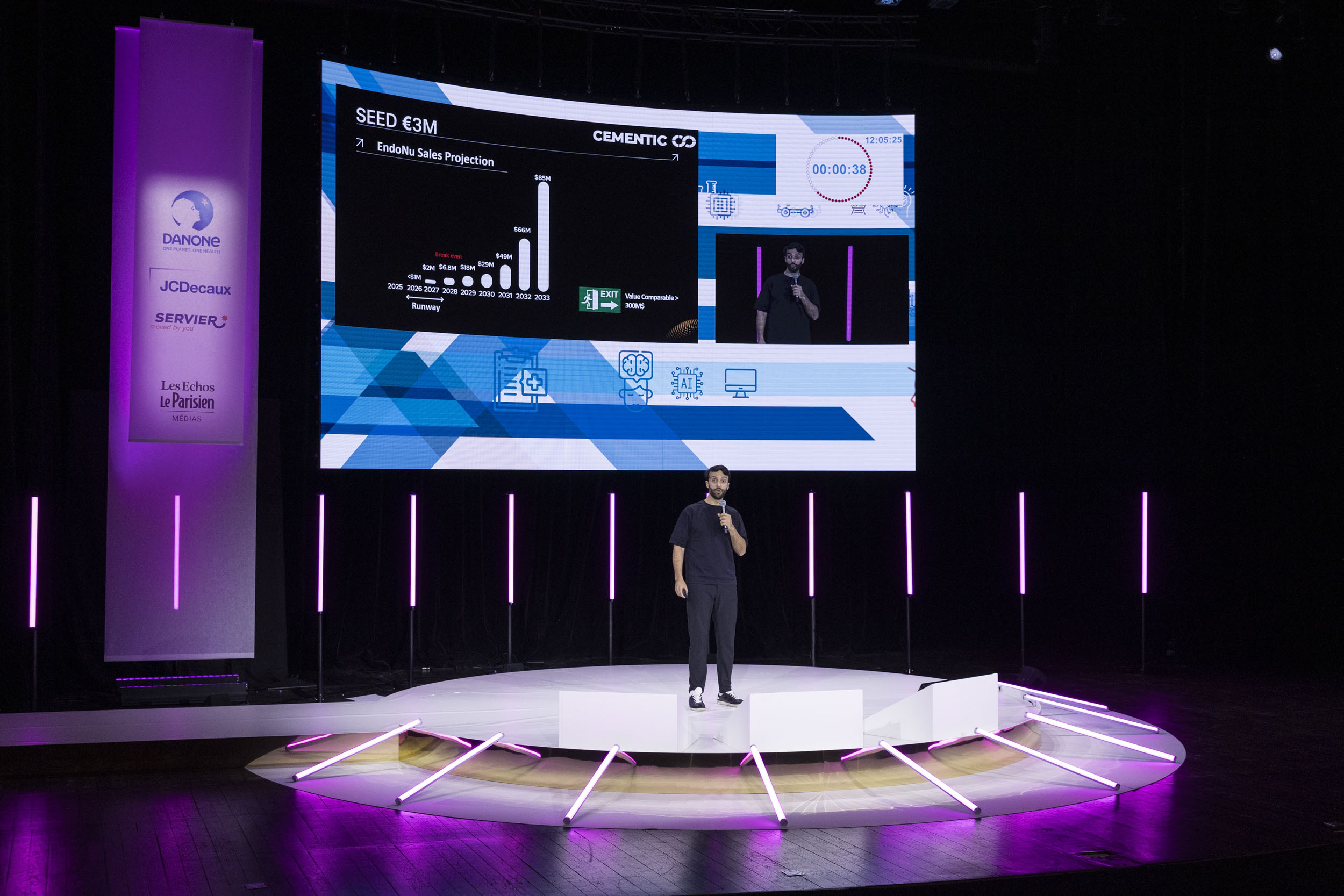
Projected 3 Year Growth
Numbers are derived from a Business Case made with FKG SA ( market penetration of FKG SA, if Henry Shein distribute higher numbers are expected)
Revenue Model
Cementic’s financial plan anticipates robust growth driven primarily by EndoNu™ sales. Wehave deliberately excluded the introduction of new products or team expansion, as operations are largely outsourced. This projection demonstrates how a lean team can achieve significant revenue with our most advanced product, underlining the sustainability of our business model. Naturally, each potential opportunity discussed above will be evaluated through an updated Business Plan to determine profitability
Revenue Model
Core revenue comes from EndoNu™ sales to distributors ($14/unit). Additional revenue can come from product diversification or services.
Adoption Asumptions :
Starting in 2026, following FDA clearance, we project conservative adoption based on a business case developed during the FKG–Cementic partnership. Importantly, this forecast treats EndoNu™ as a standard bioceramic sealer and does not account for its unique antimicrobial innovation. For context, the BC sealer launched in
Pricing & Reimbursement
EndoNu remains at a retail minimum premium price, but it is not prohibitive, with potential for private insurance reimbursement based on clinical value and cost-effectiveness. If we secure insurance reimbursement, EndoNu could become a blockbuster like the dental product Arestin.
Competitors
Cementic’s first-mover advantage and strong patent position make direct competition unlikely in the short term. Most of R&D department of our comperitior are only focuse on material sciences. We monitor biotech research closely. Our commitment to continuous innovation means that if competitors enter the market, we will likely have advanced formulations ready (e.g., faster healing, combining LNPs with growth factors or orther antiseptic molecules). We may explore partnerships or acquisitions as defensive strategies if a major competitor develops similar solutions. Many alternatives, such as GentleWave, complement rather than rival us. If new methods lower infection rates differently, EndoNu™ would still be valuable for addressing remaining bacteria. We’ll adapt our marketing to position EndoNu™ as part of a comprehensive solution alongside other advancements.
Traction
We have signed an LOI with Henry Shein (US company) and FKG SA (Swiss company).
We are in discussions with Septodont (French company) and Edge Endo (US company).
We have a partnership with Produits Dentaires (Swiss company) and Angelus (Brazilian company).
Due Diligence Docs
Please note that access to the company's confidential materials is limited. Click this button to request access from the Company and its representatives.
Updates
No updates found .
Supporters
Help us find best new ideas to fund by telling us what you think. Your feedback goes straight to the team behind this project in private, so tell them what you really think.
Index Score
1
Score
0
Score
1
Likes0
Partners0
Pilots1
Investors-
This campaign has ended but you can still get involved.See options below.
Help us find best new ideas to fund by telling us what you think. Your feedback goes straight to the team behind this project in private, so tell them what you really think.



