About our project
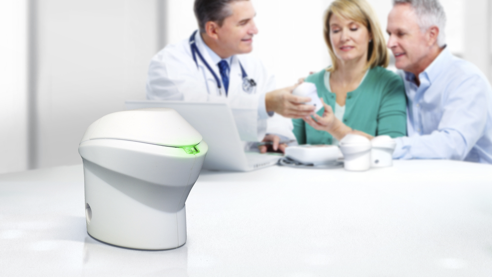
The problem we solve: Remaining engaged in mental health treatment is highly correlated with adherence to medications. Staying at home makes treatment that much more challenging. Even with telemedicine, patients are missing 25% of sessions. Published research indicates 25-40% of mental health patients stop taking medications and become unstable resulting in ER visits, hospitalizations, loss of housing, and mortality. While agencies monitor prescriptions for renewals and use pill packing to help patients, no ability to monitoring daily pill-taking leaves patients on their own to be adherent; resulting in non-compliant patients going into crisis and disengaging from treatment. They require personnel time and effort to restore them. Too many patients in crisis lead to high staff stress and high turnover rates, currently around 40% in agencies. Case managers and patient disengagement mean shrinking revenue for agencies. An agency with 4000 patients may be losing up to $7.5 million in revenue from crisis.

About our solution:
We are proposing the field testing of our medication management platform consisting of an Internet-of-things (IoT) pill dispenser and an app used by the patient and managed by mental health professionals through a cloud-based interface connected to the agency's EMR. Patients use of the IoT pill dispenser sends daily medication-taking data to the platform. The data enables identifying non-compliant patients and assigning case managers to intervene. The data provides opportunities for additional agency revenue activities including med reviews, interventions, and keeps patients in weekly therapy.
iRxReminder's IoT device or "pod" uses our patent-pending smart alerting and effortless data recording when the medication is dispensed. We use a dosing window so that if you take the medication on-time no alert is generated. But if you haven't taken your medication, then an alert is sent to your smart environment (watch, phone, tv, speaker, caregiver, etc.). Our studies show 80%+ adherence.
 Progress to
date:
Progress to
date:In Brief Our technology is validated and ready for our first field test to prove our reimbursement model. A memorandum of understanding is in place to execute a validation study with Charak Health and Wellness in Cleveland OH. We are in the process of raising the funds to manufacture the IoT dispensers and manage the study. A successful outcome will prove 1) the return on investment for license and equipment (5X for the agency) and demonstrate 2) better patient engagement and outcomes, 3) improved staff satisfaction and retention, and 4) increased agency revenue (estimated at 40% increased reimbursement revenue for patients using the technology).
Technology is complete The iRxReminder platform technology development is now complete. We validated the complete platform at the University of Michigan Cancer Center under a project funded by the Hemotology Oncology Pharmacy Association (HOPA).
The app has been developed, validated, and tested with the iOS and Android OS. The apps are available in the Apple App Store and Google Play Store. The pod is ready for manufacture at scale. We have molds ready and manufacturing can be ramped up within 6-weeks with our partner Parallel Design Inc. located in Wooster, OH. Electronics design is optimized and ready for manufacture through our partner LogiSync in Avon Lake, OH.
We are in the final efforts to complete FDA Class II clearance submission which is anticipated to be complete and in the review process with completion of our safety tests. Our FDA clearance effort is led by Dr. Fred Ma, an experienced FDA consultant with experience in bringing over 200 products through FDA clearance.
App improves adherence Sterns et al. (Sterns, 2017; NIH Grant No. 1R43AG033500) conducted a pilot to improve medication compliance using smartphones for older adults (Mean Age = 65) recovering from a stroke. Participants used the iRxReminder system or kept paper diaries for 2 months. Overall, participants using iRxReminder complied with 83% of medication events. No additional interventions, such as calls from the research team, or behavioral reinforcements, such as display of counts of successful events, were provided. Patient recall of medication adherence was poor: the smartphone group underestimated their adherence and the dairy group overestimated their adherence, showing the value of providing accurate data to the healthcare team. No one misplaced a smartphone, and half of the participants asked to keep the smartphone, demonstrating that even older adults will accept this technology.
mHealth meets telepsychiatry. The iRxReminder platform can be conceptualized as an advance in telepsychiatry that uses mHealth. As such, all of the advantages of telepsychiatry apply, such as cost effectiveness (e.g., reduced travel for follow-up meetings), increased access for patients in rural or hard-to-reach areas (e.g., prison, detox units), improved coverage of underserved groups (e.g., minorities), and improved efficiency. With respect to efficiency, iRxReminder can be used to manage large numbers of people quickly, and direct limited resources for interventions to re-stablize patients at the beginning of non-adherence. iRxReminder will also provide a quantitative assessment that may improve sensitivity and thus early detection of side effects that lead to non-adherence. Currently, there is no leading technological solution for assessing, monitoring and intervening with patients taking medications.
Cognitive prosthetic. The platform when deployed will act as a cognitive prosthetic to support successful self-management of patients living with chronic health and mental health issues. The system provides an effective monitoring system that can trigger early interventions, alerting and bringing to bear available resources just when needed. The platform can be right-sized so that those who use the system successfully are matched to the available healthcare professional team. The system will provide the quantitative information required to improve the quality of care and ensure that best practices are being added to the platform as developed. The envisioned platform provides exactly the patient-centered care one imagines is possible with technology-supported care coordination but has yet to be realized.
The data collected provide additional reimbursement opportunities for agencies and healthcare organizations. Additional activities include additional medication reviews (monthly), monitoring of medication taking, and intervention actions. Significantly, because patients are stopped from entering crisis, they are engaged in planned weekly therapy at full levels maximizing benefit to the patients and reimbursement to the agency.
About Our Team

Creator: Anthony Sterns
Location: Ohio
Education: CUNY SPS, UMUC, University of Akron
Bio: Anthony Sterns founded iRxReminder after 10-years of research on caring for people living with dementia. The key lesson gained from this work is that people lose their independence due to failing to take their medications correctly. In addition, healthcare professionals are disconnected from what their patients are experiencing and cannot share their expertise and help. iRxReminder's core purpose is to empower patients and healthcare professionals to manage medications together, to achieve high adherence, and to provide effortless clinical monitoring to enable timely interventions that lower ER visits, and improve health outcomes.
Dr. Sterns is a recognized national expert in the area of gerontechnology and a pioneer in the application of mobile computers and smartphones for improving the quality of life for individuals living with chronic illness. Dr. Sterns has over 20 years experience in designing and bringing to market products and services for the silver industries marketplace. He is a member of the APA Division 20 board and a Fellow of the Gerontological Society of America. Dr. Sterns is a board member of the International Society of Gerontechnology where he also serves as the Deputy Secretary General and as an Associate Editor for the Society’s journal, Gerontechnology.
Dr. Sterns has led National Institutes of Health grants and contracts in the development of software for medication adherence on mobile devices since 2001. His direct project experience includes serving as the principal investigator developing a post-transient ischemic attack (mini-stroke) intervention (NIH 1R43AG033500) and working on two projects with co-investigator Dr. Hughes on a management of congenital heart failure intervention (NIH 1R43AG042230) and a medication adherence system that uses artificial intelligence to monitor Tardive Dyskinesia delivered using smartphones (NIH 1R43MH114763).
Hospital Affiliation: Kent State University College of Nursing
Title: Chief Executive Officer
Advanced Degree(s): Ph.D.
About Team Members
Josh Smith
Chief Technology Officer, MBA
Biography: Josh Smith serves as the Chief Technology Officer for Creative Action LLC and for iRxReminder LLC. Josh's prior experience includes senior programming positions at IBM and IBM Watson.
Josh has worked with Drs. Anthony Sterns on previous grant-funded projects related to developing mobile applications to provide cognitive intervention therapies with older adults living with heart failure (NIH 1R43AG042230), living with dementia, and recovering from transient ischemic attack (NIH 1R43AG033500).
Josh has spent his career helping users identify their goals and achieve the IT results they want. He is an Agile practitioner (software always internally tested and in a running state), and is comfortable working with both technical and non-technical people in an iterative design process that helps involve and engage project stakeholders. Josh has extensive full software lifecycle experience in online media and mobile products (including 3 games specifically for Apple’s iPad and development of the iRxReminder, iRxCapture, and ESmCapture smartphone applications). In addition, his diverse academic background has provided a toolbox that is well stocked to handle a full range of software systems development projects. Lastly, he brings the proven leadership of successful cross-functional software and product development teams.
Title: Chief Technology Officer
Advanced Degree(s): MBA
LinkedIn:
https://www.linkedin.com/in/joshuabsmith/
Fred Pollock
Chief Business Officer, BS
Biography: Fred Pollock, has worked in the biotechnology space for 20-years with direct healthcare experience with patient stratification of genetic testing.
With all technology components in place, Fred Pollock, in the position of Chief Business Officer, just joined the team to provide leadership in revenue generation and creation of our sales organization. Fred was connected by a mutual friend after a recent exit.
Title: Chief Business Officer
Advanced Degree(s): BS
LinkedIn:
https://www.linkedin.com/in/fredrick-pollock-736a45/
How We Help Physicians
Mental health agency case managers leave school expecting a caseload of 20-30 patients they can see weekly or bi-weekly. Today caseloads are often over 80 individuals and visitations stretch out to once every other month. The inadequate attention means more patients reach a crisis that results in losing employment, losing housing, hospitalization, or incarceration. These crises require agency personnel time and paperwork to document and correct the situation. This takes away time from treatment (20 hours for 1 crisis vs. 1-hour increments of support for 20 individuals) and increases stress on caseworkers. As a result, caseworkers have high turnover, leaving the organization with an even greater caseload problem. The CDC reports that of the 43 million people with chronic and persistent mental health problems, 44% or 19.2 million people are in treatment. Mental healthcare agencies are struggling with successfully delivering services to these people due to personnel and funding shortfalls. Through participation in the NIH I-Corp program, we interviewed over 100 mental healthcare administrators, healthcare professionals, and social workers who shared with us these challenges.
How We Help Partners
A caseload of 80 patients could be given to a senior case manager and a new case manager to work together. Our NIH I-Corps interviews validated that 50% of current patients have smartphones so 40 patients can be monitored by our iRxReminder technology and the remaining individuals can be visited with weekly. Currently, an agency with 4000 patients has a revenue potential of $5200/patient or about $21 million. With 25% (1000) patients in crisis the average per patient revenue is closer to $3900. At the agency level is a $5 million loss. Turnover and too high patient loads leads to another $2.5 million in lost revenue for a total revenue reduction of $7.5 million for the agency. For those patients utilizing iRxReminder's technology the potential revenue is $7250 per patient. The higher revenue comes from additional reimbursement from medication reviews, intervention activities, full therapy engagement, and from monitoring.
The IoT pill dispensers are filled by the pharmacy services that support the mental health agency. Many agencies have in-house pharmacy services and these are the initial agencies we are now targeting.
Innovation Details
Intellectual Property Summary
iRxReminder and our development partners have US and Canadian design and utility patents issued and pending around this proposed technology. In August we completed our office actions and our patented claims were issued on December 3, 2019.
Patent Link
http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO2&Sect2=HITOFF&p=1&u=%2Fnetahtml%2FPTO%2Fsearch-bool.html&r=1&f=G&l=50&co1=AND&d=PTXT&s1=Sterns.INNM.&s2=Medication&OS=IN/Sterns+AND+Medication&RS=IN/Sterns+AND+Medication
Clinical Information
We have received IRB approval to proceed with pilot testing this methodological approach from the University of Michigan and Kent State University for specific projects. There are several projects with published articles and presentations which demonstrate the technologies' clinical efficiency.
iRxReminder Summa Health System Pilot - Improved Medication Compliance
Keynote Presentation –Sterns, A. A. (2011, April). Using smartphone technology for chronic care self-management: a new strategy for healthcare management. Invited address of the Cellar Conference, Francis Payne Bolton School of Nursing, University Center on Aging & Health, Case Western Reserve University, Cleveland, OH.
Recently, Sterns et al. (Grant No. 1R43AG033500, PI Sterns, A.) conducted a pilot to test improving medication compliance using smartphones for older adults (Mean Age = 65) recovering from a stroke (Sterns et al., 2010). Overall, participants complied with 83% of medication events, implying 9 or more missed doses in a 2-month period. No additional interventions such as calling or behavioral reinforcements such as display of counts of successful events were provided. When asked if the individual had recorded when they took medications 77% indicated yes in the experimental group and 67% indicated they had in the control group. About 33% of the experimental group indicated they recalled missing doses and indicated that 1.3 times they had missed over the 2-month period. In the control group 44% said they recalled missing doses and indicated they missed 1.8 times over the 2-month period. These pilot outcomes show that older adults are capable of using smartphones to track medication adherence, that they are significantly better at recording taking medications with the smartphone than a diary. Also both groups missed significantly more pills than they remembered demonstrating the importance of in-the-moment data collection.
Gerontologist. 2005 Dec;45(6):828-34.
Curriculum design and program to train older adults to use personal digital assistants.
Sterns AA. Creative Action LLC, Akron, OH 44303, USA. drtone@gmail.com
Abstract
PURPOSE:
The aim of this study was to demonstrate that many older adults can share in the potential benefits of using a personal digital assistant (PDA), including using the device as a memory aid for addresses and appointments, to improve medication adherence, and as a useful organizational tool and communication device.
DESIGN AND METHODS:
A curriculum, designed specifically for older adults, was developed that provided the necessary information and practice to use the technology. The degree to which the curriculum improved user skills was measured by testing participants on basic and advanced features of each of the standard PDA programs.
RESULTS:
Participants were successful in using the technology and indicated satisfaction with the medication-reminder program specifically designed to accommodate the needs of older adults.
IMPLICATIONS:
The PDA, supported with well-designed software and well-executed training, can provide unique benefits to older adults.
Sterns, A.A. (2012, June) Succeeding in mHealth in the USA. A paper presentation as part of a symposium New Technologies in Health Care at the 2012 International Society of Gerontechnology meeting, Eindhoven, The Netherlands.
Sterns, A.A., Hughes J., and Goldstein, C. (2012, June) Getting the US mHealth market to take our medicine. Gerontechnology. A paper presentation as part of a symposium Expanding e-Health Knowledge at the 2012 International Society of Gerontechnology meeting, Eindhoven, The Netherlands.
Sterns, A. A. (2011, December). Smartphone-based comprehensive self-care management for chronic conditions. Part of the Chronic Disease Management symposium at the 3rd Annual mHealth Summit, Washington, DC, December 5, 2011.
Sterns, H. L. and Sterns, A. A. (2011, November). An update on training, technology, and older adults. Part of a symposium presented to the Gerontological Society of America annual conference, Boston, MA, November 21, 2011.
Sterns, A. A. and Hughes, J. (2011, November). Smartphone-enhanced self-management and active aging. Part of a symposium presented to the Gerontological Society of America annual conference, Boston, MA, November 19, 2011.
Sterns, A. A. (2011, October). Improving clinical research performance with mHealth technologies. A presentation for research grand rounds at the Northeastern Ohio Medical (NEOMED) University, Rootstown, OH, October 28, 2011. Link: http://neomediaweb.neomed.edu/mediasite/Viewer/?peid=8c1db8e93e2d41b8a101392e573fb87d1d
Sterns, A. A. (2011, July). Improving clinical research performance with mHealth technologies. A presentation to the Northeast Ohio Network of research, Northeast Ohio Universities College of Medicine, Rootstown, OH, July 14, 2011.
Sterns, A. A. (2011, April). Using smartphone technology for chronic care self-management: a new strategy for healthcare management. Invited address of the Cellar Conference, Francis Payne Bolton School of Nursing, University Center on Aging & Health, Case Western Reserve University, Cleveland, OH.
Sterns, A. A. & Hughes, J. (2011, March). Smartphone-based Comprehensive Self-care Management for Chronic Conditions. A presentation made at Driving the Future 11: Driving the Future in Today’s Uncertain Healthcare Environment in Cleveland, OH, March 7, 2011.
Sterns , A. A. (2010, October). Managing the development of healthcare products for seniors; Understanding the business of gerontechnology. Invited address for the University of Utrecht, Netherlands October, 3, 2010.
Sterns, A. A., Lax, G, Sterns, H., and Allen, K, and Hazelet, S. (2010, May). Improving chronic care management: An iPhone application for post-stroke recovery. Paper delivered at the International Society of Gerontechnology conference in Vancouver, BC, Canada.
Sterns, A. A., Lax, G, Sterns, H., Allen, K, Hazelet, S., and Fosnight, S. (2009, November). Improving Chronic Care Management: An iPhone Application for Post-Stroke Recovery. Part of a symposium on Technology presented at the 62nd annual meeting of the Gerontological Society of America, Atlanta, GA.
Regulatory Status
The iLidRx pill dispensing device and the iRxCapture App and iRxControl Center have completed FDA 513g actions and we are currently preparing for required electronic and magnetic testing and submission for 510(k) Class II clearance. Testing can begin once our current round of funding is complete.
How we will use the funds raised
Study Overview. The iRxReminder project will occur in four stages. Each stage is described briefly here.
In Stage I we will prepare the materials and procedures for filling and conducting medication management with health organization and pharmacy partners. Following IRB approval those materials and procedures will then be tested locally where we can observe participants and healthcare professionals to ensure the procedures and materials work as intended.
In Stage II we will recruit 100 local patients through a regional healthcare service and complete the intake interviews. Local psychiatrists will be recruited, trained, and tested for mastery. Once trained, they will complete evaluations of participants’ for using the technology. Success represents the completion of Aim 1: Recruit 100 patients and paired healthcare professionals for a demonstration project
In Stage III the platform will be deployed and used for 100 participants. Success represents the completion and use for 6-months of monitoring 100 patients for one specific medication using the pod and all patient medications using the app.
In Stage IV the iRxReminder platform data will be analyzed to determine adherence, patient stability compared to historical data, and satisfaction with the platform by participants and healthcare professionals. The participants and healthcare professionals will be debriefed with a structured interview and results will be used to plan modifications to the app interface in a follow-on project. Success represents the completion of Aim 3: Test the iRxReminder smartphone delivered self-management intervention will be evaluated. Results will support the expansion of services through contracts in Phase II follow-on.
Thank You
I have a lifelong commitment to healthcare and cognitive solutions for older persons. As CEO of start-up iRx Reminder, I have innovated an internet-connected device for more advanced medication management used in research, clinical trials and personal prescription maintenance, reducing the overall cost of research, healthcare, and re-hospitalization.
One key innovation is that medication is managed together among the consumer, the healthcare professional, and the family caregiver, providing maximum transparency in adherence to the regimen. The iRx Reminder solution uses cloud- and mobile-based assistance to a state-of-the-art pill dispenser that controls and tracks medication taking. The platform reminds the consumer only when the optimal pill- taking window of time is closing. This empowers the person to take medication on time themselves, relieving caregivers and making it less likely the consumer will abandon medication management systems altogether. Sterns has applied decades of experience to universal solutions for keeping retirees healthy.



 Progress to
date:
Progress to
date:































