by Linh T. Le
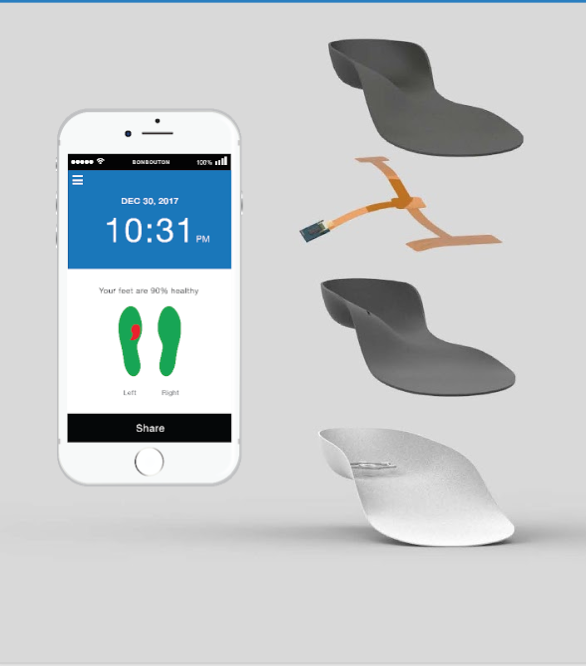
Bonbouton smart insoles are embedded with a proprietary graphene sensing system that passively monitors the skin’s physiological signals to detect early signs of foot ulcers in diabetic patients
Long Island City, NY United States AMIA challengeAbout our project
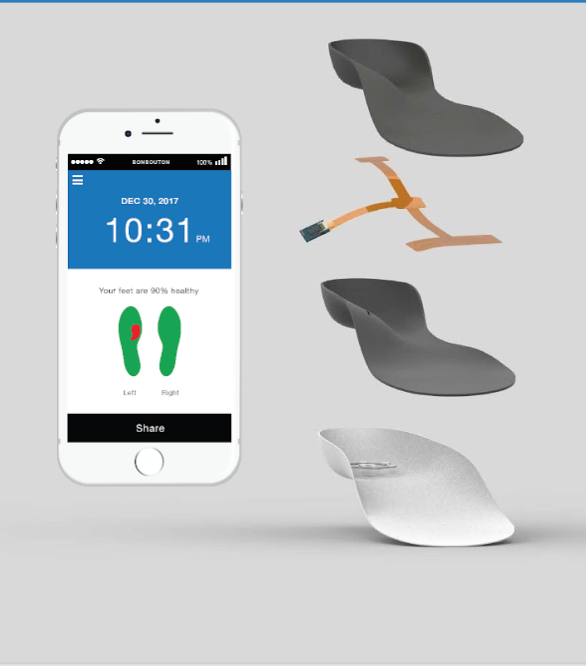
The problem we solve:
About Our Team

Creator: Linh T. Le
Location: New York
Education: PhD granted from Stevens Institute of Technol
Bio: Oversees all business development, grant funding and technical work. His background in scientific research of graphene production methods led to multiple patents, published papers, conference proceedings, and related awards.
Title: CEO & Founder
Advanced Degree(s): Ph.D (ABD)
About Team Members
Fatima Awan
Vice President of R&D, BS, MS
Biography: Directs the development and optimization of graphene sensors, scientific research, and all other R&D initiatives. She holds a MASc in Chemical Engineering and BSc from the University of Waterloo, in Canada.
Title: Vice President of R&D
Advanced Degree(s): BS, MS
Andrew Madison
Design Strategist, BA, MA
Biography: Andy runs Bonbouton’s strategic innovation practice. By conducting research and making sense of the current landscape, he helps Bonbouton meet the demands of a complex and fast-changing marketplace. He is an expert skier.
Title: Design Strategist
Advanced Degree(s): BA, MA
Olivia Schreiber
Community Engagement Strategist , BS, MA
Biography: Olivia is Bonbouton’s eyes on an uncertain and complex landscape. She engages with our stakeholders by venturing into communities and delivers critical user input needed to refine our solutions.
Title: Community Engagement Strategist
Advanced Degree(s): BS, MA
About Our Company
bonbouton
Location: 29-10 Thomson Avenue
7th floor, St 10
Long Island City, NY 11101
US
Founded: 2015
Website: http://www.bonbouton.com
Twitter: @Bonbouton
Facebook: http://www.facebook.com/bonboutonnewyork
Product Stage: Prototype/MVP
Employees: 3-5
How We Help Patients
Having high blood glucose levels is not the only complication that results from having diabetes—this disease comes with many burdens, for patients, caregivers, and physicians alike. Type I and Type II diabetics are counseled to self-inspect feet for changes in morphology, drainage and other cardinal signs of foot ulceration; however, complications such as peripheral neuropathy, obesity, or decreased mobility impose challenges to self-inspection. At Bonbouton, we are striving to remove these challenges and improve the outcomes of the estimated 30,000,000 diabetic patients living in the United States, 25% of which will go on to develop foot ulcers which in turn can lead to lower limb amputations, loss of mobility, and overall decreased quality of life. Our solution, a smart insole with a proprietary-embedded graphene sensing system, passively monitors skin biosignals to detect foot ulcers in diabetic patients. Along with a mobile application companion, patients, physicians, caregivers, and others within an individual’s health network will now have the peace of mind that the feet are being “watched” by another set of “eyes,” embedded seamlessly within the shoe insole. Data generated by the insoles will improve communication and care with providers, build better relationship between providers and insurers, and and in turn, improve overall coverage, something Americans all want.
Conducting customer discovery interviews with people living with diabetes has helped our team understand the challenges diabetes inflicts upon a person's entire healthcare ecosystem, which might include spouses, neighbors, children, and other key stakeholders. We are developing our mobile phone application to incorporate the necessary social features that will enable positive, direct communication so that fear need not be a reality for any person touched by diabetes.
How We Help Physicians
Previous research has shown that skin temperature monitoring reduces the risk for diabetic foot ulcerations in high-risk patients. Skin infrared thermometers and scales with temperature sensing elements have been introduced into the market, however they are time-intensive, impractical methods used to monitor foot health status. Bonbouton's smart insole and mobile application companion will allow patients, caregivers and providers to monitor foot health status in real-time, enabling earlier preventive treatment that can significantly reduce the cost of care and facilitate better patient outcomes.
Our usability study conducted in partnership with HITLAB as part of the Digital Health Breakthrough Network included five podiatrists, 40% of which were satisfied with their patient’s ability to monitor their feet. They identified a number of challenges, like mobility issues and neuropathy, that prevent patients from effectively self-monitoring. 80% of podiatrists agreed that the Bonbouton system could reduce time-consuming (and costly) emergency procedures by catching ulcer development early and anticipate that patients will be more motivated to make behavior changes if the mobile application allows them to visualize their risk. All of the podiatrists agreed that adopting a new, innovative technology platform such as Bonbouton would be an effective marketing tool, would help convincepayers to authorize surgical procedures or cover
orthotic inserts with patient-generated temperature data, and would ideally obtain merit-based reimbursements if the system could show their care management has lead to improved outcomes.
Following a clinical study to validate our solution, we look to apply for FDA Class I Medical Device clearance via the 510(k) pathway and to acquire the CMS codes for Medicare/Medicaid reimbursement. The patient-generated temperature and pressure data will also be subject to remote review, and with new CPT code 99091 rules in place, providers will be able to code for remote monitoring care services once per patient per month during the same 30-day service period as any of the CPT codes for CCM, TCM, and BHI.
" placeholder="" maxlength="2000">
Type I and Type II diabetics are counseled to self-inspect feet for changes in morphology, drainage, and other cardinal signs of foot ulceration; however, complications such as neuropathy, obesity and loss-of-mobility impose challenges to self-inspection. Foot ulcers that develop but go undetected can fester unbeknownst to the patient, and by the time the patient visits the podiatrist or healthcare provider, salvaging the toe, foot or entire lower limb is impossible. Over 200 amputation surgeries performed each day costing the American healthcare system $15B per year, which makes the challenge of diabetic foot ulceration one of great importance.
Previous research has shown that skin temperature monitoring reduces the risk for diabetic foot ulcerations in high-risk patients. Skin infrared thermometers and scales with temperature sensing elements have been introduced into the market, however they are time-intensive, impractical methods used to monitor foot health status. Bonbouton's smart insole and mobile application companion will allow patients, caregivers and providers to monitor foot health status in real-time, enabling earlier preventive treatment that can significantly reduce the cost of care and facilitate better patient outcomes.
Our usability study conducted in partnership with HITLAB as part of the Digital Health Breakthrough Network included five podiatrists, 40% of which were satisfied with their patient’s ability to monitor their feet. They identified a number of challenges, like mobility issues and neuropathy, that prevent patients from effectively self-monitoring. 80% of podiatrists agreed that the Bonbouton system could reduce time-consuming (and costly) emergency procedures by catching ulcer development early and anticipate that patients will be more motivated to make behavior changes if the mobile application allows them to visualize their risk. All of the podiatrists agreed that adopting a new, innovative technology platform such as Bonbouton would be an effective marketing tool, would help convincepayers to authorize surgical procedures or cover
orthotic inserts with patient-generated temperature data, and would ideally obtain merit-based reimbursements if the system could show their care management has lead to improved outcomes.
Following a clinical study to validate our solution, we look to apply for FDA Class I Medical Device clearance via the 510(k) pathway and to acquire the CMS codes for Medicare/Medicaid reimbursement. The patient-generated temperature and pressure data will also be subject to remote review, and with new CPT code 99091 rules in place, providers will be able to code for remote monitoring care services once per patient per month during the same 30-day service period as any of the CPT codes for CCM, TCM, and BHI.
How We Help Hospitals
Our solution not only serves patients and providers, but also hospitals and other medical institutions. Hospital readmissions are costly and prevalent which has driven Medicare to implement incentives to reduce hospital readmissions. As hospitals continue to incur increasing costs as the number of diabetic foot ulcerations admissions increase, there is a strong need to implement tools for diabetic foot ulcer prevention. For patients who have already experienced a diabetic foot ulcer, the Bonbouton smart insoles will enable them to monitor their foot status in real-time as well as providers to remain proactive in foot health management. The patient-generated temperature and pressure data will enable earlier intervention, thus sparring hospitals and other medical institutions the costly penalities associated with readmissions.
Studies have shown that intential management of diabetic foot ulcers reduces hospital costs and utilization. Diabetic foot health management has come to include devices, patient education, custom footwear, and patient monitoring--Bonbouton's smart insoles with connected smart phone application is one such tool that can be integrated into these types of management plans that can save the hospital precious time and resources.
We are in the process of launching our first clinical pilot to validate our solution, and we believe this would be a great opportunity for hospitals to explore a new technology. Many hospitals within the NYC-metro region are dedicated to bringing innovations such as ours to fruition, and we invite any hospital network to contact us to see how Bonbouton might dually improve patient outcomes and shed positive light on your dedication to entrepreneurial endeavors.
How We Help Partners
The Bonbouton smart insoles are just one of the many potential applications of our proprietary, inkjet-printed graphene sensors. As a flexible nanomaterial, we envision graphene sensors integrated a variety of different mediums, from shirts for monitoring heart rate and temperature to athletic clothing for EMG. Thus, there exists a great potential for partners to harness the power of graphene along with us. Companies that produce clothing should be interested in this technology, as we envision clothes as the natural physical interface of the body to measure vital signs in the near future.
One of the critical next steps is piloting so that we may have the appropriate data before filing for FDA Class I Medical Device approval. We are looking to engage within the New York City community, given its extensive network of hospital systems, private practices, and community-based organizations that have the mission to improve the lives of those living with diabetes. An element of our solution is to empower people living with diabetes to take control of their health and for those organizations that struggle to make this a reality for those they serve, we offer a new tool that can make it happen.
There is also potential for industrial partnerships. We have made several partnerships in the past year, and are continuously looking for ways to expand our network. Bonbouton recently announced a partnership with W.L. GORE Associates for a research collaboration initiative to explore material solutions in advanced graphene sensor technology and enable practical smart fabrics for assistive apparel and digital health. Additionally, we’ve entered into a joint development agreement with a U.S based advanced materials manufacturer, Liquid X Printed Metals, to take the graphene technology from prototype to production for several industrial applications.
Challenge Mission
Affiliation(s)
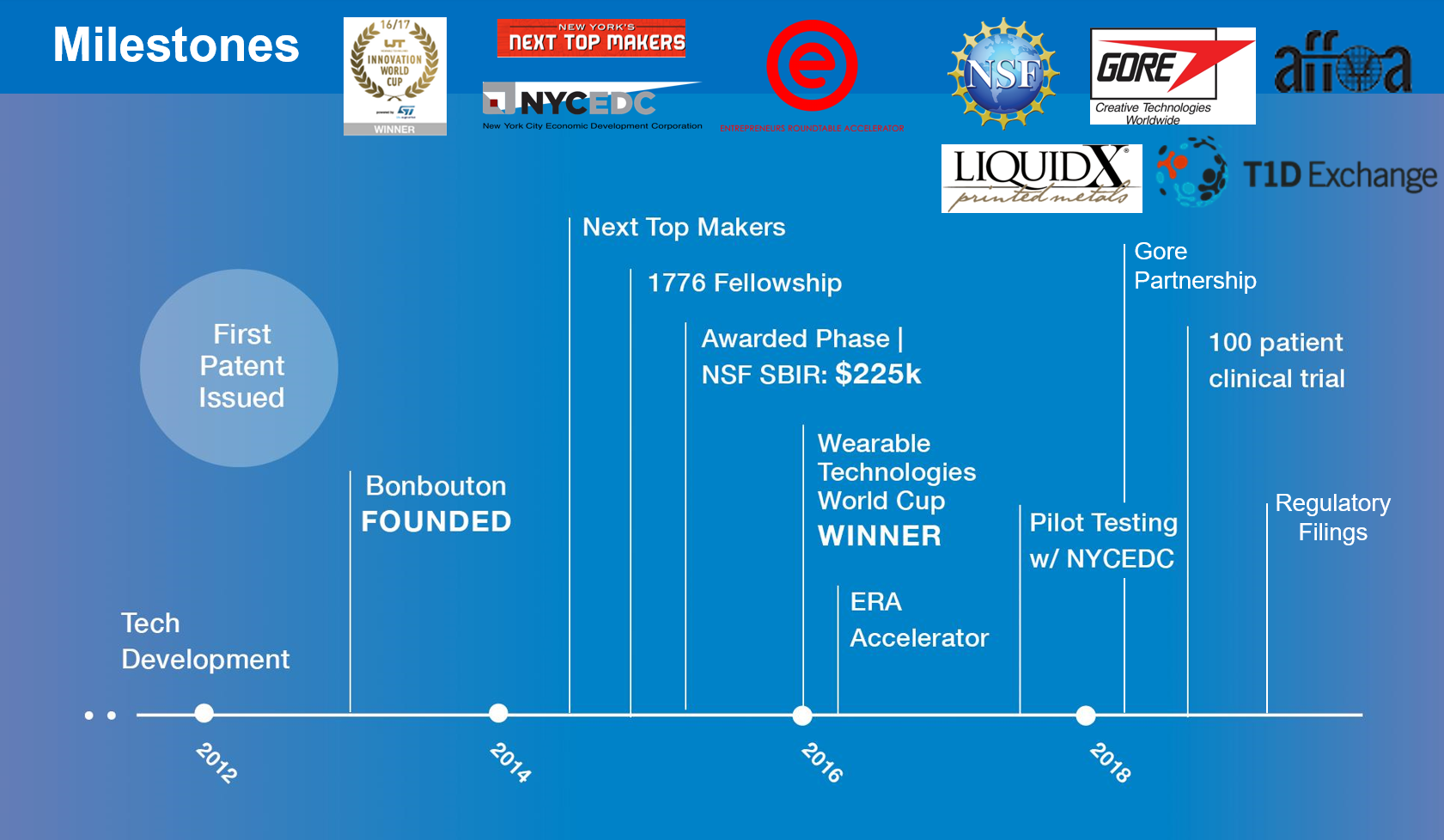
N/AKey Milestones Achieved and Planned
In addition to raising $1M in funds, being accepted into a prestigious NY-based accelerator, and forming strategic partnerships, we completed our first usability at the end of 2017 which has prepared us for an upcoming clinical pilot study of our diabetic foot ulcer solution. Over the course of the next 6 months we will work with hospitals and larger healthcare networks to monitor the feet of their high-risk patients and assess the ability of our smart insoles to detect foot ulcer warning signs. This clinical study will have two objectives: to validate the efficacy of our smart insoles and to receive feedback from patients in order to iterate on the product design. After our clinical study we’ll be submitting our technology for FDA Class II Medical Device Clearance. Once the device has been granted clearance we’ll be able to partner with established diabetic shoe/insole companies and sell our insoles through their established sales channels and distribution networks.Our Competitive Advantages
We have identified number of active companies offering smart insoles and foot monitoring technologies, but Bonbouton designates its direct competitors on the basis of 3 defining factors. These factors are value proposition, relevant geographic market, and end user. Bonbouton has classified 3 companies as direct competitors based on these defining features: Podimetrics, Siren Care, and Orpyx Medical Technologies. Our graphene-based sensor technology is expected to perform better than competitors due to the inherent flexibility and conformal properties of the nanomaterial. Our insole technology will be better applicable for skin-compatibility due to its flexibility and conformability, and none of the products on the marketplace today have this capability. The software communication tool provided through the Bonbouton platform is unlike anything on the market, enabling continuous monitoring and real time notifications when the system detects temperature and pressure discrepancies betweeBarriers to Entry
The core technology of the inkjet printing of graphene sensors was developed during CEO & Founder, Linh Le's PhD at Stevens Institute of Technology. This technology was co-developed with the U.S. Army and enables the fabrication of next-generation of sensors utilizing graphene nanomaterial in the most economical way. There are 7 issued patents and 2 pending as of October 2018 to protect the technology. Patent Link http://www.freepatentsonline.com/8810996.html http://www.freepatentsonline.com/9178129.html http://www.freepatentsonline.com/9399580.htmlFunding, Partners and Alliances To Date
To date, Bonbouton has raised approximately $1M raised so far, including $10K NYCEDC grant, $100K ERA Seed, $225K NSF Phase I SBIR Grant, $25K Tamer Funds for Social Ventures Grant, $65K in angel investments and additional cash awards/prizes. The grant from NYCEDC enabled us to conduct a usability study in partnership with HITLAB, an organization that offers digital health research, advisory, and teaching services to improve health delivery around the world. We have made several strategic partnerships, as listed before, including W.L Gore and Liquid X Printed Metals. We are aiming to have a product in market and available to NYC-area providers starting in mid-2019 and expand regionally later in 2019. We also plan to offer our smart insoles paired with Quikiks Hands-Free footwear for diabetic patients so that we can take advantage of pre-existing diabetic footwear and insole distribution channels.Innovation Details
Intellectual Property Summary
The core technology of the inkjet printing of graphene sensors was developed at Stevens Institute of Technology. This technology was co-developed with the U.S. Army and would enable the fabrication of next-generation of sensors utilizing graphene nanomaterial in the most economical way. There are 7 issued patents and 2 pending as of October 2018 to protect the technology.
Clinical Information
As a participant of the NYCEDY Digital Health Breakthrough Network, we performed a usability study in partnership with HITLAB (n=14, 9 patients, 5 providers). Research activities were conducted at HITLAB headquarters, located in Manhattan on the Columbia University Medical Campus, and Progressive Podiatry, a private podiatry clinic located in Park Slope, Brooklyn, that served as a partner site for recruitment and participant visits. The data showed that 100% of podiatrists predicted they would use the Bonbouton system in their practice, 100% of patients indicated interest in foot health technology, and 89% of patients reported a “serious risk” of developing ulcers and a need to monitor feet daily. Bonbouton is currently in the process of raising money from venture capital investment firmswith the express goal of funding a clinical pilot study of our diabetic foot ulcer solution. Overthe course of the next 6 months we will work with hospitals and larger healthcare networks to monitor the feet of their high-risk patients and assess the ability of our smart insoles to detect foot ulcer warning signs. This clinical study will have two objectives: to validate the efficacy of our smart insoles and to receive feedback from patients in order to iterate on the product design.
We have begun the IRB approval process and are seeking podiatry offices and larger hospital networks to partner with to conduct our study.
Regulatory Status
We are currently in the process of raising funds for our first 100-patient clinical trial. After our clinical study we’ll be submitting our technology for FDA Class I Medical Device Clearance, using data from the study to support our application. Once the device has been granted clearance we’ll be able to partner with established diabetic shoe/insole companies and sell our insoles through their established sales channels and distribution networks. Bonbouton expects to realize revenues by early 2020.
How we will use the funds raised
With additional funds, we look to address 3 areas: manufacturing, clinical and regulatory strategy, and marketing/sales. We look to improve the insole prototype by focusing on material selection for comfortability and manufacturing process compatibility, prototyping to hit such targets, testing small scale manufacturing, and developing the smart phone application for remote monitoring. Refinement of the prototype is a critical step as we prepare to begin our first 100-patient pilot usability study within 9 months. While testing patient outcome and effectiveness, we will need to direct attention to regulatory filings research/strategy and Medicare reimbursement codes. We hypothesize that the implementation of our preventative monitoring tool with significantly reduce the need for foot amputations and the five-year mortality rate in the diabetic patient group. Thus, a compatible, comfortable, and scalable prototype, with additional funds will add great value to the study, which in turn will support our 510(k) submission for premarket clearance with the FDA. Following this clearance, we will proceed with direct marketing to consumers, projected for early 2020.
Thank You
Bonbouton is a powerhouse team of innovative thinkers and boundary-pushers. We are all dedicated to bringing our diverse expertise to the problem of diabetic foot ulcers. Our entire team has been touched by diabetes, whether it be a loved one or a good friend. We have heard from people living with diabetes and their struggles to manage their foot health in addition to their physical, mental, and emotional well-being. These struggles need not be a reality for anyone, and that’s why Bonbouton is reimagining diabetic foot health management. With your help, we can empower people living with diabetes to take control and step into a healthier future. Please join us.
Updates
No updates found .
Supporters
-
 Instant Feedback
Instant Feedback
Help us find best new ideas to fund by telling us what you think. Your feedback goes straight to the team behind this project in private, so tell them what you really think.
40Medstartr
Index Score40
Interest
Score0
Adoption
Score3
Likes0
Partners0
Pilots0
Follows-
This campaign has ended but you can still get involved.See options below.
$ 10,000 goal
Instant Feedback
Help us find best new ideas to fund by telling us what you think. Your feedback goes straight to the team behind this project in private, so tell them what you really think.
-




