RiverTown BioSciences LLC: A new treatment for lung scarring due to Covid-19 infection
RT1840 is a repurposed, safe compound, formulated as an inhaled product to directly administer to the lung to prevent/reverse scarring (fibrosis)
Dobbs Ferry, NY United States COVID-19 Pulmonary/ Asthma Equity Raise WarOnCOVID challengeAbout our project
The problem we solve: Pulmonary fibrosis is a debilitating, chronic disease that can be caused by Covid-19. There is currently no treatment for this lasting consequence of moderate to severe Covid-19 infection

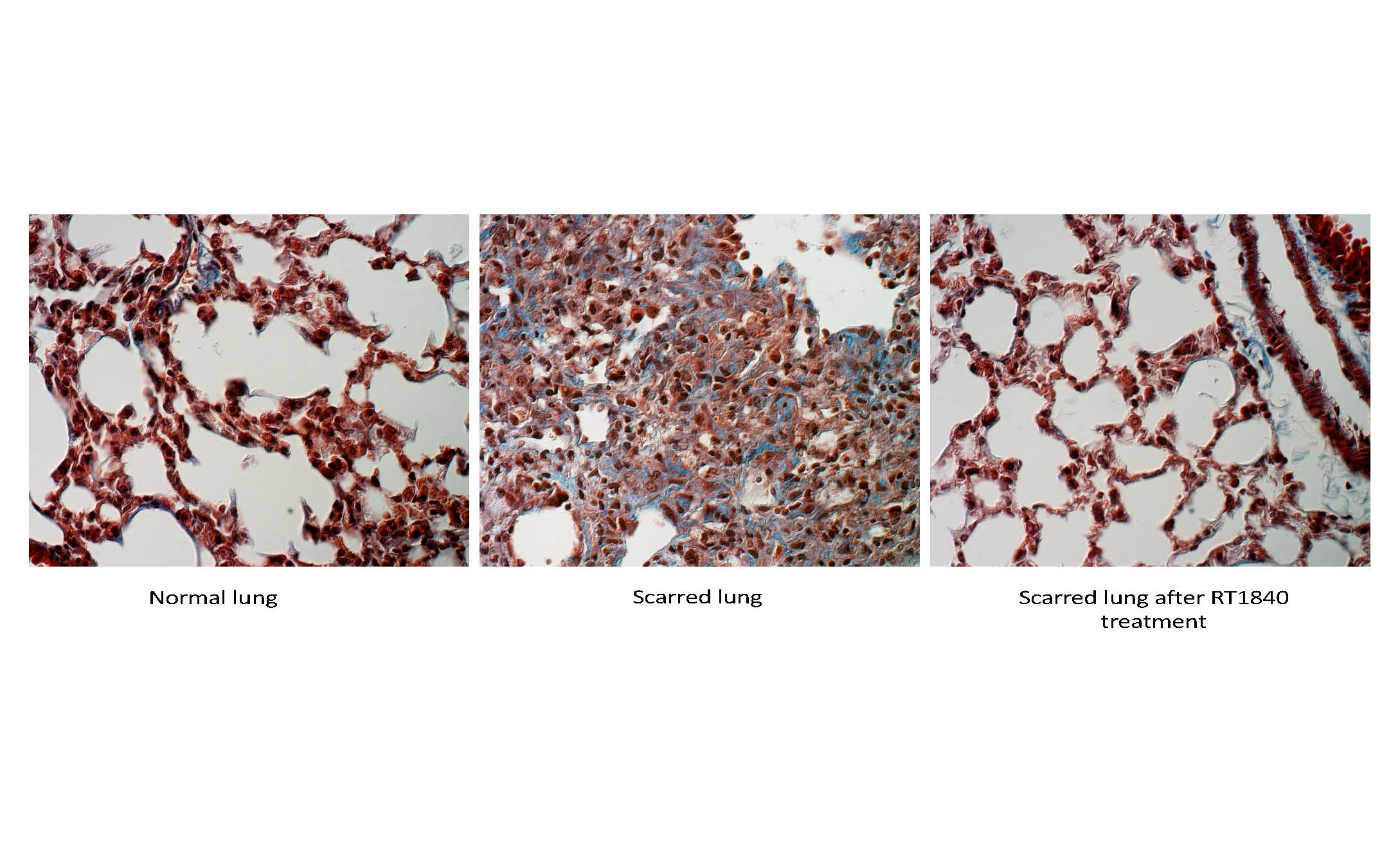
About our solution: We have developed RT1840 as an aerosolized treatment for delivery into damaged lungs. Our data show a profound reversal of lung damage with RT1840 therapy This compound has an outstanding safety profile in humans.
Progress to date:
- Reformulation of APIs with great safety profiles over hundreds of people/year exposure
- Novel mechanism of action
- Over $138MM spent on development to date
- Full IND-enabling safety, toxicology and PK/PD packages
- Strong IP on composition and formulation
- RT1840 works to regenerate normal lung in the setting of pulmonary fibrosis
About Our Team

Creator: David Weinstein
Location: New York
Bio: Neurologist and Neuroscientist; former Professor in the Departments of Neuroscience, Neurology, Pathology and the Comprehensive Cancer Center at the Albert Einstein College of Medicine and Adjunct Associate Professor of Pathology, Columbia College of Physicians and Surgeons Seasoned biotech executive, with C-suite experience at InteKrin Therapeutics, GliaMed Therapeutics, RiverTown Therapeutics, Androbiosciences MS (Pharmacology), MD, and PhD from NYU and Columbia College of Physicians and Surgeons
Title: Chief Executive Officer
Advanced Degree(s): MD, PHD
About Our Company

RiverTown BioSciences LLC
Location: 39 Round Hill Road
Dobbs Ferry, NY 10522
US
Founded: 2020
Product Stage: Prototype/MVP
Employees: 1-2
How We Help Patients
Pulmonary fibrosis is a debilitating, chronic disease that can be caused by Covid-19. There is currently no treatment for this lasting consequence of moderate to severe Covid-19 infection. We believe that RT1840 has the possibility to give Covid-19 patients the ability to ameliorate the lasting effects of the virus-induced lung damage.How We Help Physicians
It is very frustrating for physicians to be unable to fully treat patients with chronic debilitating disease. Pulmonary fibrosis is such a condition. There is currently no treatment for this lasting consequence of moderate to severe Covid-19 infection. We believe that RT1840 has the possibility to give providers that treat Covid-19 patients the ability to ameliorate the lasting effects of the virus-induced lung damage.How We Help Hospitals
It is very frustrating for physicians to be unable to fully treat patients with chronic debilitating disease. Pulmonary fibrosis is a such a condition. There is currently no treatment for this lasting consequence of moderate to severe Covid-19 infection. We believe that RT1840 has the possibility to give providers that treat Covid-19 patients the ability to ameliorate the lasting effects of the virus-induced lung damage.How We Help Partners
It is very frustrating for partners and caregivers to watch loved ones suffer from a debilitating disease Pulmonary fibrosis robs patients of air, while it limits them from participation in family life, and often interferes with self-care. There is currently no treatment for this lasting consequence of moderate to severe Covid-19 infection. We believe that RT1840 offers the opportunity to restore lung function and return patients and their families to a more active and interactive life.Challenge Mission
COVID Problem We Address
Pulmonary fibrosis is a debilitating, chronic disease that can be caused by Covid-19. Based on early data, it is eatimated that up to 80% of moderate to severe Covid patients will suffer from lung scarring. There is currently no treatment for this lasting consequence of moderate to severe Covid-19 infection. We believe that RT1840 has the possibility to give Covid-19 patients the ability to ameliorate the lasting effects of the virus-induced lung damage.
Our COVID Solution
We believe that RT1840 has the possibility to give Covid-19 patients the ability to ameliorate the lasting effects of the virus-induced lung damage, and thus return them to a fuller, more active and interactive life.
Innovation Details
Intellectual Property Summary
Patent applications are pending on the composition of matter, formulation, routes of delivery and disease indications for RT1840, as well as the API's. It is our intent to apply for world wide patent protection.
Clinical Information
- RT175, the key API in our treatment, was originally developed as an oral medication for the treatment of neural degenerative diseases, beginning in 2002
- Human clinical experience:
- 611 people exposed to doses ranging from 100mg to 6g
- Safe and well-tolerated
- 4 Phase 1 safety and PK studies
- 4 Phase 2 Studies in Parkinson Disease and post-prostatectomy ED
- Longest and highest exposure was 4g/day for 2 years po
- 611 people exposed to doses ranging from 100mg to 6g
- Over $138MM spent on development to date
- Full IND-enabling safety, toxicology and and PK/PD packages
- Based on unexpected and results in animal studies, the drug was repurposed to a low-dose aerosol to treat pulmonary fibrosis
- New formulation
- IP well protected
Regulatory Status
RT1840 has two APIs- low dose cyclosporine A and our proprietary drug, RT175.
Cyclosporine A is an approved, generic drug that has a long history as oral, parenteral and inhaled administration, It's safety at the intended dosage is well established
RT175 was developed for neurologic indications, before being repurposed here. It has been through numerous Phase 1 and 2 human trials, with an outstanding safety record at dosed up to 5 million times greater than the concentration in RT1840.
This is a phase 1B/2A ready program
How we will use the funds raised
- Build-out management team
- Regulatory
- Submission of a new IND for the Covid-19 indication
- Clinical development
- Phase 1B/2A trial
- Phase 3 trial
- NDA application
- G and A
- M and A activities
Thank You
Post-Covid lung morbidity is an unmet and growing need. RT1840 presents and opportunity to do well by doing good in a market that will have real need.
Investor Info
Market Size
- Estimated that 40-70% of the worlds population will be infected with COVID-19
- Early reports suggest that up to 70% to 80% of moderately to severly affected Covid-19 patients will have residual pulmonary disease and fibrosis. This is a huge and unmet market
Projected 3 Year Growth
We seek to either co develop or license RT1840 to a larger biotech or pharma company.
Revenue Model
RT1840 will be a prescription drug. As such, we anticipate that the salesforce will call on pulmonologists, critical care, infectious disease specialists and internal medicine physicians.
Competitors
There currently is no effective treatment for pulmonary fibrosis. To the best of our understanding, there are no treatments in development to reverse Covid-19 induced lung scarring
Traction
We have been in ongoing discussions with a number of potential equity and strategic partners
Due Diligence Docs
Please note that access to the company's confidential materials is limited. Click this button to request access from the Company and its representatives.
Updates
No updates found .
Supporters
-
07/27/2020 - Interested in investing in the project.
07/07/2020 - Interested in investing in the project.
06/08/2020 - Liked the project. , Bachelor's degree in Political Science and Government from
, Bachelor's degree in Political Science and Government from
04/28/2020 - Liked the project. Instant Feedback
Instant Feedback
Help us find best new ideas to fund by telling us what you think. Your feedback goes straight to the team behind this project in private, so tell them what you really think.
17Medstartr
Index Score17
Interest
Score0
Adoption
Score4
Likes0
Partners0
Pilots2
Investors-
This campaign has ended but you can still get involved.See options below.
$500,000 pledged of $35,000,000 goal$500,000 Investor, Pilot & Parnter
interest to date.
Instant Feedback
Help us find best new ideas to fund by telling us what you think. Your feedback goes straight to the team behind this project in private, so tell them what you really think.

