Off Patent Drugs for Covid : Off patent Drug Formulation using topical delivery for Covid
Plant based alkaloid and off patent drug combination of Ivermectin and anti-hementic drug using topical delivvery
Mumbai, CO United States COVID-19 Infectious Disease Equity RaiseAbout our project

The problem we solve: Covid is a major pandemic and effective treatment is required
About our solution: 2 of the three molecules are already proven in invitro test. We have added one more off patent drug in the formulation. The topical application is easy and effective. The plant based alkaloid inhibits viral replication along with being anti-inflammatory and off patent drugs acts on various pathways.
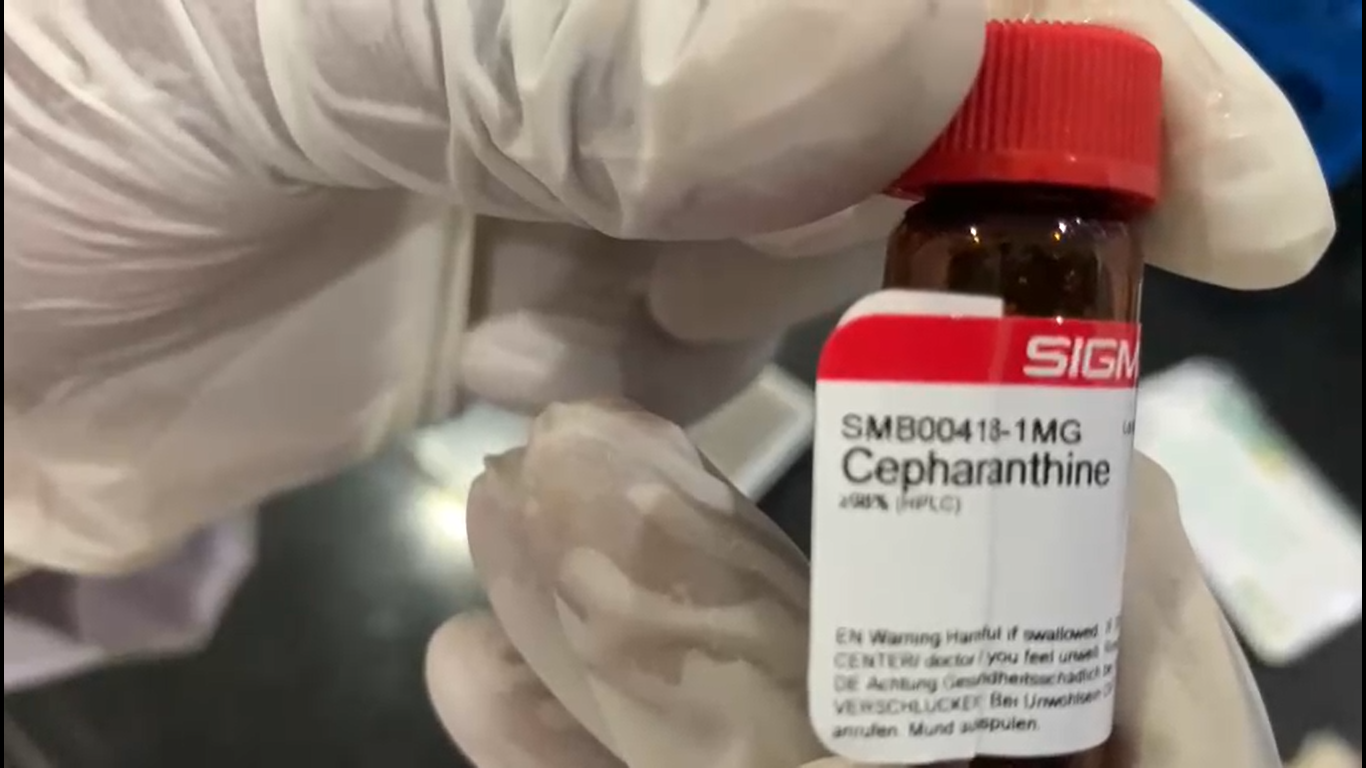
Progress to date:
Formulation ready to be tested invitro at CCMB Hyderabad for Covid.
About Our Team

Creator: Subhramani Naidu
Location: Meath
Bio: A business researcher with top tier investment banking having expertise across sectors and geographies.
Title: Project Head
About Our Company
QuickGun LifeScience
Location: 402, Mehivish Tower, Patel Complex, Mira
Mumbai, CO 401107
US
Founded: 2020
Product Stage: Prototype/MVP
Employees: 1-2
Innovation Details
Intellectual Property Summary
Provisional patent is filed in India and accepted.
Clinical Information
Cepharanthine and Ivermectin have proven effective in viral reduction invitro. Based on Ivermectin similar class of drug should enhance the combination and topical application makes easier regulatory approval.
Regulatory Status
Formulation currently in queue for invitro testing for Covid in CCMB India.
How we will use the funds raised
I plan to outsource entire clinical phase to a reliable firm and seeks emergency FDA approval. FDA approval would be easier on account proven safety of off patent drugs and topical application.
Thank You
This can formulation can be a effective in treating covid hence support us in this initiative
Investor Info
Market Size
TAM - 169,736,766 recovered patient &
Unestimated preventive market for mild and moderate cases
Projected 3 Year Growth
Estimated revenue in 3 years $15 million
Revenue Model
Sustainable revenue model by exclusive sales of product globally
Competitors
Vaccine makers and any other new treatments
Traction
Center for molecular Biology agreed to test our formulation invitro for Covid based on the molecules profile.
Due Diligence Docs
Please note that access to the company's confidential materials is limited. Click this button to request access from the Company and its representatives.
Updates
No updates found .
Supporters
There are not supporters yet.
Help us find best new ideas to fund by telling us what you think. Your feedback goes straight to the team behind this project in private, so tell them what you really think.
0
Score
0
Score
0
Likes0
Partners0
Pilots0
Follows-
This campaign has ended but you can still get involved.See options below.
Help us find best new ideas to fund by telling us what you think. Your feedback goes straight to the team behind this project in private, so tell them what you really think.