Korus: Autonomous Sleep Care
by Jong Lee
Korus is a self-repositioning bed that provides autonomous repositioning. Its versatility makes it ideal for medical issues impacted by positioning, providing relief at home or in the hospital.
Everett, MA United States Neurology Equity RaiseAbout our project
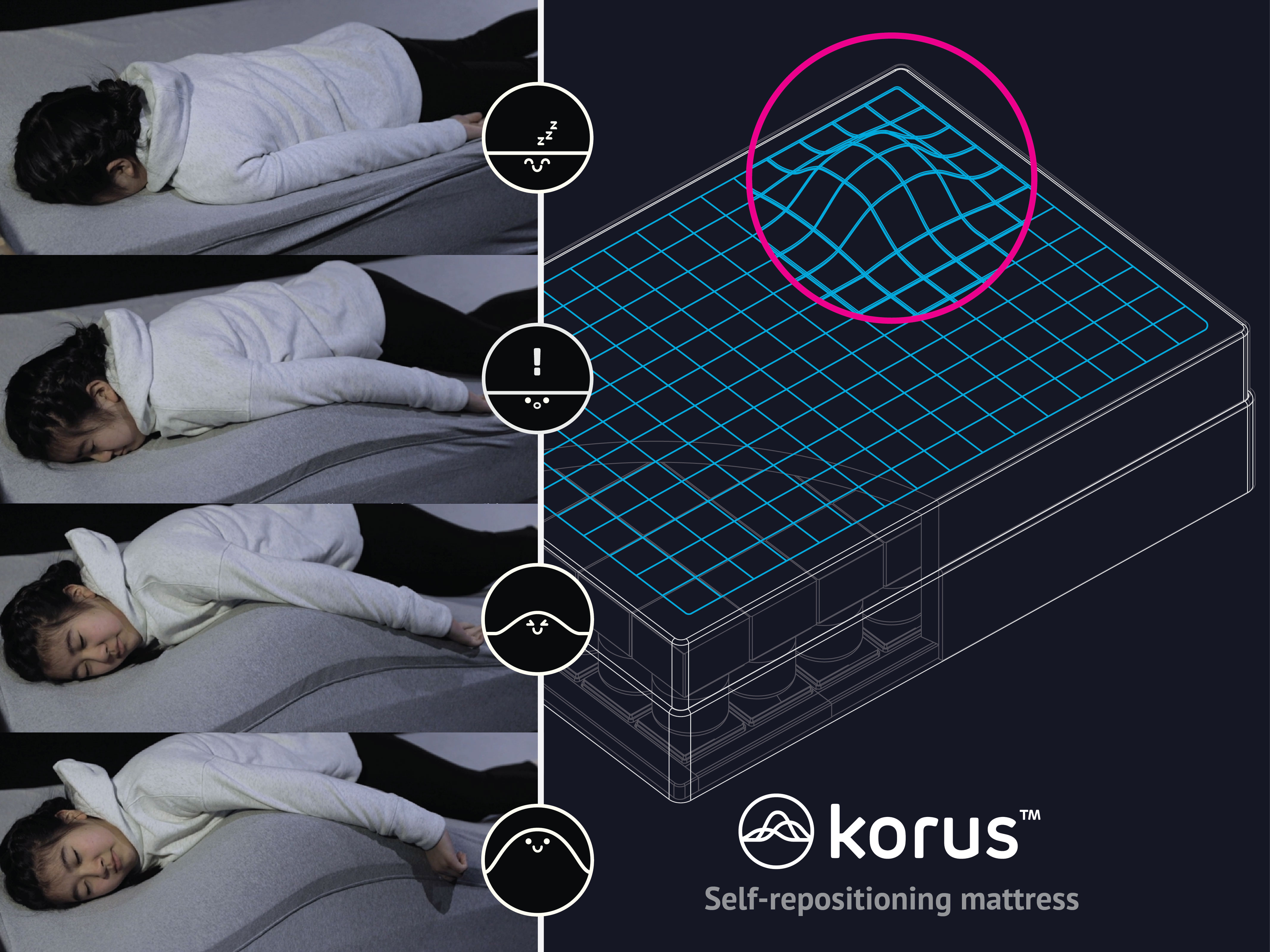
The problem we solve: The Problem Addressed: Many patients face significant challenges in bed, as they depend on others for repositioning. This issue is particularly critical for individuals with epilepsy, for whom sleeping in a prone position can tragically increase the risk of sudden unexpected death in epilepsy (SUDEP) by as much as 85%. The issue extends beyond this group, impacting a wide range of individuals, including those with disabilities, patients recovering from various conditions, and even individuals suffering from chronic snoring or back pain. There is an urgent need for an innovative solution that can autonomously ensure optimal sleep posture, offering timely repositioning when necessary.

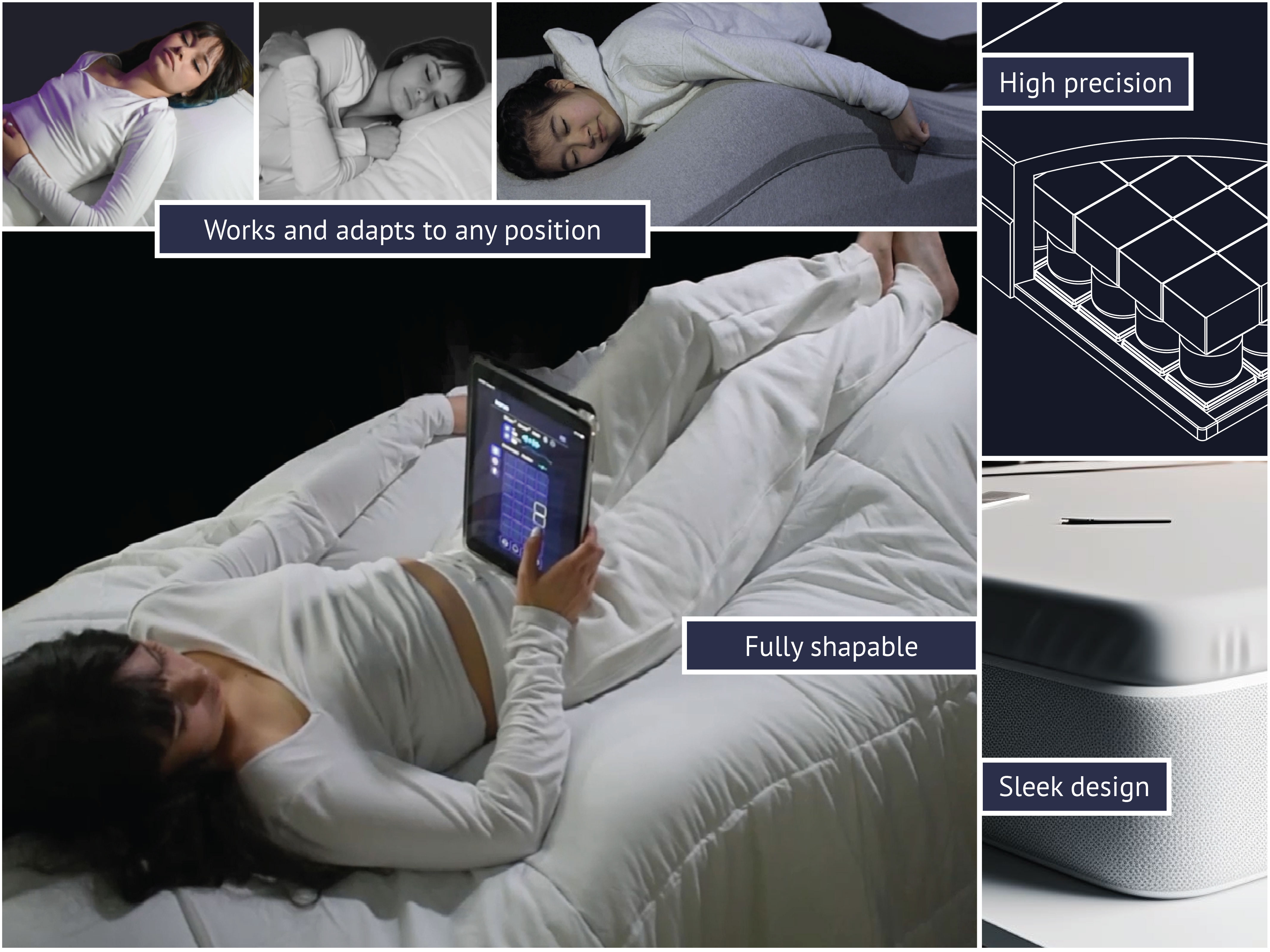
About our solution: Korus employs machine learning to adjust its surface dynamically for optimal support. It detects the sleeper's posture, adapting and shifting to enhance comfort or entirely change positions. Unlike conventional systems limited to face-up positions, Korus operates in any sleeping orientation, offering unparalleled versatility. Its architecture ensures adaptability across all body types and fits most bed frames for easy implementation. Some of the benefits include: • Caregiver Relief: By automating patient positioning, Korus will reduce significantly physical and emotional strain on caregivers. • Cost Reduction: Korus is a game-changer in healthcare economics, slashing medical costs by minimizing the need for constant, hands-on patient care. • Quality of Life: By reducing dependency and facilitating switching positions, Korus will enhance the overall quality of life for its users, patients, and their families. • Versatile Health Benefits: Korus can respond to multiple health issues.
Progress to date:
Our progress today includes:
- We've developed all systems and achieved a beta prototype.
- Successfully integrating a comprehensive system with Android and iOS apps and our inaugural machine learning application.
- Completed an IRB-approved medical study on repositioning and position recognition at Brigham and Women's Hospital, here are the results: https://doi.org/10.1101/2024.01.02.23300653.
- We can generate data for ML training, testing, and further product development.
- From 2020 to 2022, we secured approximately $650K in non-dilutive funding, including a $503K SBIR Phase 1 grant from the NIH and $125K from the Epilepsy Foundation via their EF Shark Tank, underscoring robust support from key medical and epilepsy advocacy groups.
- We've proposed an SBIR Phase 2 grant to obtain an additional $3 million in non-dilutive funding bolstered by a matching offer from a strategic investor for another $3 million.
- Authorization to initiate patient registry for our first clinical trial, marking a pivotal step toward commercialization.
- Our progress is also propelled by a distinguished technical team focusing on machine learning, complemented by a seasoned advisory board comprising experts including Arthur Maxwell (Business and manufacturing), Eamonn Hobs (Regulatory and development), Steven Cohen (Corporate legal), Reg B. Wilcox III, PT, DPT, MS (Rehabilitation medicine), and Dr. Paul R. Albear, MD (Pressure injury prevention). This collective expertise ensures Korus is not just a product, but a comprehensive solution poised to redefine sleep care.
About Our Team

Creator: Jong Lee
Location: Massachusetts
Education: Harvard Medical School
Bio: Dr. Lee Jong Woo, an innovator in neurology, focuses on epilepsy and neurophysiology. A Harvard alumnus with a Computer Science A.B. (1993), his blend of technology and science led him to McGill University, where he completed a Ph.D. in Neuroscience (1999) and M.D.c.M. (2001). His postgraduate journey included an Internal Medicine internship at the New York Hospital of Queens and a Neurology residency at Partners Healthcare, Boston. Specializing further, he completed a Clinical Neurophysiology fellowship at Brigham and Women's Hospital, Boston. As an Associate Professor at Brigham and Women's Hospital, Harvard Medical School, Dr. Lee's work centers on epileptic patient care, brain tumor-associated seizures, and pioneering medical devices. His development of CT and MRI-compatible EEG leads has transformed ICU EEG procedures. A leader in professional societies like the American Academy of Neurology, his accolades include the Medical Research Council of Canada MD/PhD Fellowship and the Dreifuss EpiFellows award, highlighting his research and clinical achievements. Dr. Lee's studies on seizures, particularly in critically ill patients and SUDEP (sudden unexpected death in epilepsy), have been groundbreaking. He co-invented the Korus device to decrease SUDEP risks and founded Soterya for its commercialization. His career, marked by academic rigor, clinical excellence, and a commitment to improving patient outcomes, makes him a prominent figure in neurology.
Hospital Affiliation: Associate Professor of Neurology Division of Epilepsy Brigham and Women’s Hospital
Title: Co-founder & Medical Director
Advanced Degree(s): Ph.D
About Team Members
Lina Williamson
Co-founder & Co-CEO, DVM Ph.D
Biography: Lina has held various prestigious positions throughout her career, including Group Leader at Novartis Animal Health and Director at the Novartis Institutes for Biomedical Research. Her leadership roles have spanned across continents, from Switzerland to Boston, and now to her entrepreneurial and advisory roles in Seattle and Barcelona. Lina's diverse background, spanning industry, academia, and government, has equipped her with a unique perspective on innovation assessment and development.
Her contributions to science include understanding the molecular and cellular mechanisms of Pemphigus vulgaris and the pathomechanisms of skin diseases using animal models. Lina's work has been recognized with multiple awards, including the "Klingman Award for Outstanding Work of a Young Scientist" by the Society of Investigative Dermatology.
Lina's involvement in the community and professional organizations further reflects her commitment to advancing medical science and education. From fundraising support for Colombian children in Boston to advising on Mexico's COVID-19 response, her efforts extend beyond the laboratory and into the heart of communities in need.
As Lina continues her fellowship at MIT's Catalyst Program, her journey is a testament to the power of dedication, innovation, and the relentless pursuit of translating research into solutions that address some of the most challenging medical issues of our time. Her work not only advances the frontiers of dermatology but inspires a new generation of scientists and entrepreneurs to follow in her footsteps, impacting global healthcare.
Title: Co-founder & Co-CEO
Advanced Degree(s): DVM Ph.D
LinkedIn:
https://www.linkedin.com/in/lina-williamson-leisey/?originalSubdomain=es
Andres Rodriguez
Co-founder & Co-CEO, Product Design
Biography: Andres, an inventive entrepreneur, blends industrial design, robotics, biomedical technology, and product innovation. He has Industrial Design, Electronics, and Simulation expertise and is dedicated to improving lives through creativity and technical skill. Starting with Eveling, a mattress company, Andres demonstrated his entrepreneurial flair and innovative problem-solving. His career diversified significantly in 2014 at Emulate, Inc., where he designed organ-mimicking cell culture systems, marking a leap in biotechnology. His stint at Hasbro, Inc. as an associate inventor merged design with toy manufacturing to boost children's cognitive development.
As Soterya's co-founder and CEO, Andres co-invented its flagship technology, incorporating robotics and machine learning, and played a pivotal role in UI/UX design and product development. Under his leadership, Soterya secured $650k in pre-seed funding, won the Epilepsy Foundation's Shark Tank (2020), and received the SBIR phase 1 award (2021). As Co-CEO, he's leading a seed funding round, securing a $3 million investor match, and pursuing an SBIR phase 2 for $3 million.
Andres has also significantly contributed to drug delivery and laboratory instrument design, including an insulin pen and microfluidics devices. His work in scientific visualization and simulations has been recognized, earning him the Excellence in Innovation Award from Partners Healthcare in 2019. Through his diverse achievements, Andres exemplifies innovation, leadership, and a commitment to health and technology advancements.
Title: Co-founder & Co-CEO
Advanced Degree(s): Product Design
LinkedIn:
https://www.linkedin.com/in/andres-camelo/
About Our Company

Soterya, Inc.
Location: 167 Bow St.
202 E
Everett, MA 02149
US
Founded: 2020
Website: https://www.soteryabio.com/
Product Stage: Prototype/MVP
Employees: 3-5
How We Help Patients
Korus revolutionizes patient care by autonomously repositioning individuals during sleep to ensure optimal breathing and reduce risks associated with immobility, such as pressure sores and sleep-related disorders. Its intelligent design adapts to different body shapes and sizes, offering personalized support and mitigating conditions like back pain and snoring. For epilepsy patients, Korus's quick-response mechanism can shift a person from a prone to a safer position, potentially decreasing SUDEP risks. This innovation promises independence for patients and relief for caregivers, paving the way for safer, more comfortable healing and rest.
How We Help Physicians
Korus is engineered to address a critical gap in patient care: the need for constant, manual repositioning of patients to prevent complications like pressure ulcers and to aid those with conditions such as epilepsy. This autonomous system utilizes advanced machine learning algorithms to detect a patient's position and engage a dynamic pneumatic mechanism, adjusting the sleeping surface in real-time to ensure optimal positioning. For providers, this means reducing the physical burden on staff, minimizing the risk of injury from manual patient handling, and freeing up resources to focus on other critical care tasks.
Furthermore, Korus's innovative technology supports improved patient outcomes by reducing the risks associated with immobility and improper positioning, such as pressure injuries and sleep-related complications. By maintaining consistent movement and optimal positioning throughout the night, Korus enhances the overall quality of care.
Integrating seamlessly into existing healthcare infrastructures, Korus is designed for compatibility with standard bed frames, making it a practical and cost-effective solution for healthcare providers. With a proven track record backed by successful clinical studies and substantial non-dilutive funding, Korus stands as a reliable, forward-thinking investment in patient health and provider efficiency.
By choosing Korus, providers will not only see an improvement in patient care but also experience a reduction in costs associated with long-term complications. This aligns with both patient-centric care models and the financial goals of healthcare facilities, making Korus an invaluable addition to modern healthcare services.
How We Help Hospitals
Korus introduces a groundbreaking solution that addresses a critical challenge faced by hospitals and medical institutions worldwide: the need for constant, manual patient repositioning to prevent pressure injuries, enhance recovery, and ensure patient safety during sleep. Traditional patient care requires significant manpower for repositioning, especially at night, placing a strain on resources and increasing the risk of staff injuries from lifting and moving patients.
Korus leverages advanced machine learning algorithms to autonomously adjust and reposition patients without human intervention, reducing the workload on healthcare staff and minimizing the risk of occupational injuries. This technology is particularly crucial for patients with conditions like epilepsy, where improper sleep positioning can lead to severe consequences, including SUDEP (Sudden Unexpected Death in Epilepsy). By autonomously moving patients from risky positions, Korus significantly enhances patient safety.
Moreover, Korus's ability to adapt to various body types and positions ensures personalized care, improving sleep quality and aiding in faster recovery. Its implementation can lead to a reduction in pressure injuries—a common and costly problem in healthcare settings—by ensuring optimal pressure distribution throughout the night.
For hospitals, integrating Korus means elevating the standard of care while optimizing operational efficiency. It allows medical institutions to allocate their resources more effectively, focusing on critical care and other essential tasks. Additionally, by reducing the incidence of pressure injuries, hospitals can lower the costs associated with prolonged hospital stays and treatments, directly impacting their bottom line positively.
In essence, Korus presents a win-win solution for medical institutions: enhancing patient safety and comfort while significantly reducing the physical and financial strains on the healthcare system. Its adoption marks a step forward into a future where technology and healthcare merge to offer smarter, safer, and more efficient patient care.
Innovation Details
Intellectual Property Summary
Soteria's intellectual property strategy is anchored by its national phase PCT patent (US20220330892A1), which safeguards the innovative Expandable smart Cell, the architecture of the mattress, and the methods for repositioning patients using the bed. Although, the smart cell is the cornerstone of the Korus mattress's technology. This cell is pivotal, with its ability to pneumatically expand 15 inches in the Z-axis while detecting motion, pressure, temperature, sound, and humidity. Zooming up, the core elements protected by the patent include the telescopic body, a multilayer cushion or pad, an integrated flat sensor within the pad, and a motion terminal that manages airflow, cell valve operation, and data processing from the sensor, along with the unique design of the cell, mattress, and their manufacturing and repositioning methods.
To complement the patent, Soteria is taking a strategic approach to protect its proprietary algorithms by keeping them confidential rather than registering them. This ensures that the critical technological differentiators remain exclusive to Soterya. The company also trademarks the Korus brand, which will secure its brand identity and protect its market presence. This multi-faceted approach to intellectual property, blending robust patent protection with the strategic secrecy of key algorithms and brand trademarking, positions Soteria to safeguard its innovations and maintain its competitive advantage.
Patent Link
https://patents.google.com/patent/US20220330892A1/en?oq=US20220330892A1
Clinical Information
Korus shows promising benefits for several medical issues. However, our primary goal is to develop a night-time seizure management system to lower the risk of sudden unexpected death in epilepsy (SUDEP). Here is the basis of our research.
Convulsive seizures during sleep and prone position are a major risk factor for SUDEP. About 70% of SUDEP occurs after a nocturnal generalized tonic-clonic seizure (GTCS)1–3 due to greater cardiorespiratory instability during sleep, postictal airway obstruction, and increased likelihood of being alone.4 Up to 87.6% of patients are found in the prone (face-down) position after a nocturnal SUDEP1,5, even though the incidence of post-convulsive prone position is low (4.2-7.2%).6,7 Central apnea3,8,9 and autonomic instability10–12 have been identified as key risk factors for SUDEP. Prone sleeping position blunts arousal responses and reduces heart rate variability, potentially contributing to both.13–15 The relative risk of SUDEP in the prone vs non-prone position following a GTCS is over 60-fold.16
SUDEP can be prevented by repositioning and stimulating the patient. Patients can be saved from SUDEP by repositioning to avoid the postictal prone position: 1) Sudden Infant Death Syndrome (SIDS), the closest analogous disorder to SUDEP sharing many pathophysiologic features17–19 and similar risk with prone position20,21; has been reduced by up to 75% through “back to sleep” prevention campaigns.20,22–24 2) Every SUDEP documented by video-EEG has died in the prone position.3,25–27,16-18 Asphyxia from airway obstruction and hypoventilation due to the patient’s prone positioning has been documented on video-EEG.25 3) At home, merely having nocturnal supervision or a bed partner decreases the risk of SUDEP28–31, suggesting that simple interventions such as repositioning and stimulating the patient substantially decrease risk.
The timing of intervention is critical. In convulsions leading to SUDEP, terminal asystole and apnea occur as soon as 25 sec postictally and in virtually everyone by 180 sec3. Intervention must occur during this time. One patient died of SUDEP wearing a seizure detection device (considered best in class) that was functioning flawlessly; he was found prone in bed despite help arriving in 15 minutes.32 Autonomous intervention is therefore imperative. There are currently no methods to deliver interventions within the required period.
Korus has a first-in-class potential to intervene to prevent SUDEP autonomously. Korus will continually monitor patients during sleep, detect the post-convulsion prone position, and reposition/stimulate patients to the optimal recovery (sideways) position. It will provide precision monitoring and autonomous intervention. This overcomes the critical challenge that most patients with SUDEP are found unattended, and many patients do not have bedroom-level supervision. Korus was developed after careful examination of multiple potential solutions, including wearable vests, smart belts, and other designs. The final design was chosen with a patient focus group organized by the Epilepsy Foundation of New England.
Main References
1. Ali, A. et al. Association of sleep with sudden unexpected death in epilepsy. Epilepsy & Behavior 76, 1–6 (2017).
2. Lamberts, R. J., Thijs, R. D., Laffan, A., Langan, Y. & Sander, J. W. Sudden unexpected death in epilepsy: people with nocturnal seizures may be at highest risk. Epilepsia 53, 253–257 (2012).
3. Ryvlin, P. et al. Incidence and mechanisms of cardiorespiratory arrests in epilepsy monitoring units (MORTEMUS): a retrospective study. Lancet Neurol 12, 966–977 (2013).
4. Alexandre, V. et al. Risk factors of postictal generalized EEG suppression in generalized convulsive seizures. Neurology 85, 1598–1603 (2015).
5. Liebenthal, J. A., Wu, S., Rose, S., Ebersole, J. S. & Tao, J. X. Association of prone position with sudden unexpected death in epilepsy. Neurology 84, 703–709 (2015).
6. Wang, S. et al. The incidence of peri-ictal prone position in patients with generalized convulsive seizures. Epilepsy and Behavior 61, 158–161 (2016).
7. Shmuely, S., Surges, R., Sander, J. W. & Thijs, R. D. Prone sleeping and SUDEP risk: The dynamics of body positions in nonfatal convulsive seizures. Epilepsy Behav 62, 176–9 (2016).
8. Vilella, L. et al. Postconvulsive central apnea as a biomarker for sudden unexpected death in epilepsy (SUDEP). Neurology 92, e171–e182 (2019).
9. Vilella, L. et al. Association of Peri-ictal Brainstem Posturing With Seizure Severity and Breathing Compromise in Patients With Generalized Convulsive Seizures. Neurology 96, e352–e365 (2021).
10. Myers, K. A. et al. Heart rate variability in epilepsy: A potential biomarker of sudden unexpected death in epilepsy risk. Epilepsia 59, 1372–1380 (2018).
11. Nayak, C. S., Sinha, S., Nagappa, M., Thennarasu, K. & Taly, A. B. Lack of heart rate variability during sleep-related apnea in patients with temporal lobe epilepsy (TLE)—an indirect marker of SUDEP? Sleep and Breathing 21, 163–172 (2017).
12. Thijs, R. D., Ryvlin, P. & Surges, R. Autonomic manifestations of epilepsy: emerging pathways to sudden death? Nat Rev Neurol 17, 774–788 (2021).
13. Galland, B., Reeves, G., Taylor, B. & Bolton, D. Sleep position, autonomic function, and arousal. Arch Dis Child Fetal Neonatal Ed 78, F189–F194 (1998).
14. Kato, I. et al. Spontaneous Arousability in Prone and Supine Position in Healthy Infants. Sleep 29, 785–790 (2006).
15. Richardson, H. L., Walker, A. M. & Horne, R. S. C. Sleep position alters arousal processes maximally at the high-risk age for sudden infant death syndrome. Journal of Sleep Research 17, 450–457 (2008).
16. Esmaeili, B., Dworetzky, B. A., Glynn, R. J. & Lee, J. W. The probability of sudden unexpected death in epilepsy given postictal prone position. Epilepsy Behav 116, 107775 (2021).
17. Sowers, L. P., Massey, C. A., Gehlbach, B. K., Granner, M. A. & Richerson, G. B. Sudden unexpected death in epilepsy: fatal post-ictal respiratory and arousal mechanisms. Respir Physiol Neurobiol 189, 315–23 (2013).
18. Massey, C. A., Sowers, L. P., Dlouhy, B. J. & Richerson, G. B. Mechanisms of sudden unexpected death in epilepsy: the pathway to prevention. Nature Reviews Neurology 10, 271–282 (2014).
19. Richerson, G. B. & Buchanan, G. F. The serotonin axis: Shared mechanisms in seizures, depression, and SUDEP. Epilepsia 52, 28–38 (2011).
20. Guntheroth, W. G. & Spiers, P. S. Sleeping prone and the risk of sudden infant death syndrome. JAMA 267, 2359–62 (1992).
21. Ponsonby, A. L., Dwyer, T., Gibbons, L. E., Cochrane, J. A. & Wang, Y. G. Factors potentiating the risk of sudden infant death syndrome associated with the prone position. N Engl J Med 329, 377–82 (1993).
22. Gemble, A. et al. Knowledge assessment of sudden infant death syndrome risk factors in expectant mothers: A prospective monocentric descriptive study. Arch Pediatr 27, 33–38 (2020).
23. Changing concepts of sudden infant death syndrome: implications for infant sleeping environment and sleep position. American Academy of Pediatrics. Task Force on Infant Sleep Position and Sudden Infant Death Syndrome. Pediatrics 105, 650–6 (2000).
24. Mitchell, E. A., Brunt, J. M. & Everard, C. Reduction in mortality from sudden infant death syndrome in New Zealand: 1986-92. Arch Dis Child 70, 291–4 (1994).
25. Tao, J. X. et al. SUDEP, suspected positional airway obstruction, and hypoventilation in postictal coma. Epilepsia 51, 2344–2347 (2010).
26. McLean, B. N. & Wimalaratna, S. Sudden death in epilepsy recorded in ambulatory EEG. J Neurol Neurosurg Psychiatry 78, 1395–1397 (2007).
27. SJ, P., M, W.-Y. & VP, S. Sudden death in epilepsy: single case report with video-EEG documentation. Epilepsia 33, 123 (1992).
28. Langan, Y., Nashef, L. & Sander, J. W. Case-control study of SUDEP. Neurology 64, 1131–1133 (2005).
29. Nashef, L., Fish, D. R., Garner, S., Sander, J. W. A. S. & Shorvon, S. D. Sudden Death in Epilepsy: A Study of Incidence in a Young Cohort with Epilepsy and Learning Difficulty. Epilepsia 36, 1187–1194 (1995).
30. van Andel, J., Thijs, R. D., de Weerd, A., Arends, J. & Leijten, F. Non-EEG based ambulatory seizure detection designed for home use: What is available and how will it influence epilepsy care? Epilepsy and Behavior 57, 82–89 (2016).
31. Sveinsson, O., Andersson, T., Mattsson, P., Carlsson, S. & Tomson, T. Clinical risk factors in SUDEP: A nationwide population-based case-control study. Neurology (2019) doi:10.1212/WNL.0000000000008741.
32. Picard, R. W. et al. Wrist sensor reveals sympathetic hyperactivity and hypoventilation before probable SUDEP. Neurology 10.1212/WNL.0000000000004208 (2017) doi:10.1212/WNL.0000000000004208.
33. Rheims, S. & Ryvlin, P. Patients’ safety in the epilepsy monitoring unit: time for revising practices. Curr Opin Neurol 27, 213–8 (2014).
34. Sauro, K. M., Wiebe, S., Macrodimitris, S., Jette, N. & Team, E. M. U. Q. I. Quality indicators for the adult epilepsy monitoring unit. Epilepsia 57, 1771–1778 (2016).
35. Sauro, K. M. et al. Quality and safety in adult epilepsy monitoring units: A systematic review and meta-analysis. Epilepsia 57, 1754–1770 (2016).
Regulatory Status
Korus represents an innovative advancement in Epilepsy care and given its unique features without a direct predicate, is anticipated to be classified as a Class II medical device, suitable for the De Novo 510(k) submission. Our device prioritizes patient safety by autonomously shifting individuals from prone to recovery positions, addressing factors linked to SUDEP, such as asphyxiation, CO2 buildup, and chest pressure, which may contribute to systemic dysfunctions. While claiming direct SUDEP prevention is not a viable regulatory strategy—given the randomness and relative rarity of SUDEP occurrences—autonomous recovery positioning aligns closely with the strategy and mechanisms understood from extensive SIDS research. We are committed to rigorous post-market surveillance to gather vital data, which will be instrumental in substantiating our claims regarding SUDEP risk mitigation over time. Our approach is rooted in adaptability and evidence, ensuring adherence to regulatory requirements while meeting the needs of the market.
Through IRB approval and with funding from the NIH (NIH R43NS120394) Soterya performed a study to demonstrate the feasibility of developing a smart mattress to detect the prone position and rapidly repositioning subjects into a recovery position. Future development includes the development of a control system to automate repositioning based on sensor data. The completion of this device, when paired with a seizure detection device, has the potential to lower the risk of SUDEP by >50%. https://www.medrxiv.org/content/10.1101/2024.01.02.23300653v1
Soterya's strategy involves seeking the FDA's 'Breakthrough Device' status for Korus. This status would facilitate the navigation through the reimbursement pathways, particularly two: 'New Technology Add-On Payment' (NTAP) for inpatient hospital settings and the Durable Medical Equipment (DME) system for home use. To qualify for this status, Korus must meet certain criteria: A) Offer a treatment option for a patient population unresponsive to, or ineligible for, currently available treatments. B) It improves clinical outcomes significantly relative to conditions previously available, as demonstrated. At the DME level, Korus must show A) a reduction in at least one clinically significant adverse event or B) a Decreased rate of at least one subsequent diagnostic or therapeutic intervention, including a decreased number of future hospitalizations or physician visits, rapid beneficial resolution of the disease process treatment including, but not limited to, a reduced length of stay or recovery time, and an improvement in one or more activities of daily living or improved quality of life. NTAP and DME could add significant market opportunities for Korus. We have started developing collaborations to navigate these processes with experts, including Eamonn Hoobs, and consultants Accorto Regulatory Solutions and Kalms Consulting in reimbursement.
How we will use the funds raised
We plan to channel the investments primarily into three key areas:
- Product Development and Testing (70%): To finalize the development of our MVP, including refining the machine learning algorithms and ensuring that the hardware is robust and reliable.
- Scaling Manufacturing and Operations (20%): Preparing for market launch by scaling up our manufacturing capabilities, securing our supply chain, and establishing the logistics infrastructure.
- Compliance and Certifications (10%): Obtaining certifications for market entry.
Thank You
Join Us in Revolutionizing Sleep Care with Korus.
At the heart of our mission, Korus stands as a beacon of innovation, designed to significantly enhance the lives of individuals globally. The journey to develop Korus has been rigorous and demanding, but our dedication has brought this transformative technology within reach.
Our goal is to bring Korus to those who need it most. We’re reaching out for your support.
Korus is a life-enhancing solution with the potential to diminish the risks of SUDEP, particularly for our younger patients. We are on the brink of making a profound difference—improving sleep safety and quality of life, and we are confident in the promising outcomes it offers.
Your support can fuel this momentum. By joining our cause, you become a part of a community committed to making safer sleep a reality for everyone. Support Korus, and be a part of this exceptional journey towards good and safe sleep for all.
Investor Info
Market Size
We've delineated three primary segments representing significant opportunities for Korus.
- Patients Facing Bed Mobility Issues, Including Epilepsy Patients: This segment encompasses individuals with health challenges requiring bed mobility support. It includes those susceptible to pressure injuries, individuals with limited mobility due to spinal injuries, and those requiring emergency repositioning, particularly Epilepsy patients. These applications will require individual regulatory clearance.
- Individuals with Sleep-Related Issues (Entry market): This group targets customers suffering from snoring, back pain, and sleep disruption due to improper posture. Korus aims to provide a non-intrusive solution that improves sleep quality and overall health. This product variant can be commercialized as a consumer-grade with a potentially shorter time to market.
- Institutional Care and Caregivers: This category is for those assisting with patient repositioning at home or in professional healthcare settings. They are looking for reliable, automated solutions to enhance the quality of care provided while reducing.
Primary market segments:
Parents of Children with Epilepsy: SUDEP is the leading cause of death in young Epilepsy patients, creating a critical demand for dedicated care and resources to assist parents in managing their child's condition, especially at night, when the risk is higher. These families often undergo significant lifestyle adjustments and incur expenses related to SUDEP prevention, including intensive observation and emergency visits. Korus alleviates these burdens by serving as a comprehensive nighttime seizure management system for children with Epilepsy, providing essential supervision and enabling rapid intervention during seizures. Overall, Korus offers:
- Reliable Support: It functions as a proactive system, stepping in during critical moments to ensure safety and ease parental anxiety.
- Seizure Management Assistance: The bed is engineered to alleviate the physical and emotional stress of emergency seizure situations during unsupervised hours.
- Reducing Costs Related to Epilepsy Care: By reducing falls from bed during seizures and human dependency, patients, families, and payers will experience fewer incidents that require emergency visits or special supervision at night.
Subsegments:
- Patients in the initial diagnosis phase: Families in this segment face the highest costs and need support navigating the new diagnosis. Their healthcare journey often includes frequent clinic visits, a storm of diagnostic tests, and trials of various anti-epileptic drugs (AEDs). Interventions should optimize seizure control to mitigate immediate costs and improve health-related quality of life (HRQOL). In this context, the Korus mattress can play a pivotal role by offering a safer sleep environment and reducing the impact of nocturnal seizures and the risk of SUDEP while achieving seizure-free. Once a drug is assigned, Korus contributes positively to managing Epilepsy from the initial stages.
- Drug-resistant and Complex Cases: For 20%-40% of pediatric patients with drug-resistant Epilepsy, costly alternatives like Epilepsy surgery have become a necessity, demanding not only more intensive support systems and education on complex care options but also information on invasive treatments. In this context, the Korus mattress is a crucial aid, offering a non-invasive solution that can enhance patient comfort and safety, particularly in managing nocturnal seizures and reducing SUDEP risk, providing much-needed support in complex Epilepsy care.
Epilepsy adults:
Adults with Refractory Epilepsy: Adult patients who have not found relief with traditional medications or surgeries require alternative, patient-centered solutions. Our market analysis recognizes their need for innovative technologies to manage Epilepsy independently.
Subsegments:
Newly Diagnosed Adults: This sub-segment, like the pediatric one, requires considerable guidance at the onset, particularly in navigating treatment options and understanding their new condition. They may be more receptive to innovative solutions such as Korus as they seek effective management strategies.
Experienced Adults with Ineffective Medication Routines: These individuals are familiar with the healthcare system but require supplementary support to manage their condition. Solutions that can integrate into their established routines while providing additional independence can be highly beneficial.
Adults with Severe Epilepsy: Focused on individuals with complex Epilepsy types who may need specialized care. Technologies like Korus could be a valuable adjunct to their existing healthcare regimen.
Individuals with limited access to monitoring: Adults with Epilepsy navigate multifaceted challenges that extend beyond health care, affecting their lifestyle, independence, living arrangements, and even location. They require comprehensive intervention strategies that bolster their quality of life and support independent living situations.
Pricing:
The average price for a comparable consumer-grade smart mattress is around $11,500, with some offerings including a monthly subscription fee of approximately $280. In contrast, medical-grade equivalents can command prices up to $35,000. Our goal is to offer our unit at a $9,000 price point, a target we aim to achieve within the next 6 to 7 years. However, due to initial limitations, our entry price will be higher. This strategy positions us to capture early revenue, establish a premium brand image, and maximize profitability as we expand. The estimations in this assessment are based on offering the unit at $9,000.
Epilepsy Market Potential:
Total Addressable Market (TAM): The Epilepsy patient population in the United States is approximately 3.5 million. Assuming the Epilepsy market as the potential market for Korus, valued at about $31.5 billion.
Serviceable Available Market (SAM): We project that with regulatory clearance and a modest growth rate, Korus could capture 5 to 12 percent of this market within ten years post-clearance. This market share would translate to projected revenues of between $2.2 billion and $3.8 billion. Our initial focus is on pediatric Epilepsy and those patients who can directly afford Korus or obtain financing while we achieve reimbursements.
Serviceable Obtainable Market (SOM): Considering several factors, such as product scalability, our goal is to serve 110,000 patients from this segment in the next 10 years after entering the market. These targets projected revenues ranging from $1 billion to $1,5 billion.
Additional considerations: U.S. Market Focus: With about 3.5 million people living with Epilepsy in the U.S., our initial focus will be on regions with higher incidences, including California, Texas, Florida, and the Eastern U.S., representing 60% of the total U.S Epilepsy population.
Expansion Plans: We aim to expand into Canada, Australia, and Europe, which collectively have over 6,5 million patients, and Asia, with more than 23 million patients. We intend to maintain control over R&D and product experience while partnering with locals for manufacturing and logistics operations.
Entry Market Segments and Areas to Grow: (Sleep wellness market potential)
A consumer-grade Korus will primarily focus on catering to individuals struggling with sleep-related challenges due to pre-existing medical conditions. Some critical areas of action include managing snoring and back pain.
Korus addresses sleep wellness through three core factors:
- Good breathing
- Proper posture
- Holistic sleeping health monitoring
User demographics:
1. Professional Adults:
Active professional women (age from 35-68): this group encompasses dynamic professional women who regularly grapple with issues like snoring or back pain due to work-related strain, hormonal fluctuations during menstrual cycles, or physiological changes induced by pregnancy. Korus can substantially improve their sleep quality and overall well-being by providing a solution that actively adapts to their sleeping posture and offers in-bed surface pressure therapy as relief from discomfort.
Active professional men (aged 35-65): this demographic includes men regularly facing sleep disturbances like snoring and physical discomfort like back pain, typically due to occupational stressors. Korus' ability to tailor the sleeping surface to their needs and improve their sleep hygiene makes it an ideal choice for this group.
Adult Seniors (aged 66-75): This group consists of both men and women living at home, frequently encountering sleep challenges caused by health issues, such as snoring, back pain, and circulatory problems almost at a chronic level. We want to focus mainly on women in this demographic who, during and after menopause, experience a higher incidence of snoring and back pain compared to men of similar age. Korus' personalized and responsive sleeping surface can provide much-needed comfort and support, enhancing their overall sleep and quality of life.
Sleep wellness market potential overview:
Chronic Snoring
- SOM: 480 K
- Individuals with severe sleep disruption due to chronic snoring.
- Post-Menopausal women.
- Overweight or obese individuals with nasal and sinus issues.
- SAM: 16 M
- TAM: 65 M
Chronic Back pain
- SOM: 510 K
- Individuals with persistent nocturnal back pain.
- Adults with degenerative spine disease.
- Individuals with lifestyle-induced or chronic back pain.
- SAM: 17 M
- TAM: 90 M
Projected 3 Year Growth
After completing our minimum viable product, we'll be positioned to launch the first batch of 500 units, initiating a pre-ordering campaign. We anticipate collecting $1.2 million within the first three months. This initial batch is expected to generate $9.5 million in gross earnings, leading to an estimated net income of approximately $4.2 million.
Looking forward to 2025, our strategy includes selling and delivering about 2,000 units, thereby generating $38 million. We plan to start pre-orders for this second batch upon achieving 50% of our initial sales target. By the end of 2026, our objective is to sell 4,000 units, aiming to generate $76 million in revenue.
Given our initial higher pricing strategy, our focus is on offering superior returns on investment and benefits when compared to other products in the Smart and Luxury categories. Limited manufacturing capacity and access constraints necessitate higher pricing to manage demand and ensure resources for scaling and development. We estimate that, within the first three years of operation, we will have delivered 6,500 units and generated gross revenue of $110 million.
We are refining our operations to adopt a produce-to-order strategy, thereby reducing capital requirements and enhancing manufacturing efficiency. Initially focusing on low-volume, sustainable production, we aim to facilitate growth within the consumer market. The strategic introduction of our consumer-grade product is designed to establish a scalable presence, setting the stage for significant expansion into the medical-grade sector, where higher entry barriers offer a distinct competitive advantage.
Our sales targets are intentionally conservative, with a planned gradual increase in unit sales reflecting a sustainable scaling strategy. This aligns with our production capabilities. While our projections are conservative, they position us to achieve key revenue milestones, highlighting our product's market viability and underscoring our dedication to long-term profitability.
Revenue Model
Direct Sales of Unit: we will establish a strong direct-to-consumer sales channel via experience places and a strong e-commerce platform. This reduces middleman costs and allows Soterya to manage customer experience. A dynamic website with robust e-commerce capabilities would be essential, offering online customization tools for customers to tailor the Korus mattress to their preferences. Soterya will invest aggressively in digital marketing and social media engagement to connect with communities and drive sales.
Diversification via add-ons: Korus' foundational architecture remains largely uniform across applications, enabling us to offer specialized ML programs and software service packages as add-ons. These add-ons, delivered directly from Soterya's app store to the bed, can be purchased individually to meet specific customer needs and run simultaneously. For instance, Epilepsy, snoring, and back pain can be purchased separately and run simultaneously. This strategy diversifies our revenue streams and appeals to a broader customer base, enhancing user engagement and driving financial growth.
Subscription and fee-for-service model for special services: Subscription fees won't be the main source of revenue, particularly from medical segments like Epilepsy patients who need non-stop services. The subscription fee will offer additional premium services or special services related to data analytics and special health insights, including service, maintenance, and upgrades that may be added to the bid package.
Accessories and Upgrades: The modular Korus mattress enables easy customization and size updates without replacing the entire unit. This feature has proven especially appealing to parents. They can initially invest in a more affordable twin-size Korus for their young children and later upgrade to a larger size as their kids grow into teenagers. This cost-effective approach allows parents to pay only for the upgrade rather than purchasing a new bed.
Revenues from reimbursement: Soterya's reimbursement strategy involves seeking the FDA's 'Breakthrough Device' status for Korus. This status would facilitate the navigation through the reimbursement pathways, particularly two: 'New Technology Add-On Payment' (NTAP) for inpatient hospital settings and the Durable Medical Equipment (DME) system for home use. NTAP and DME could add significant market opportunities for Korus. We have started developing collaborations to navigate these processes with experts, including Greg Dale, a collaborator in Soterya's SBIR Phase 1, and consultants Accorto Regulatory Solutions and Kalms Consulting in reimbursement.
Competitors
There are several competitors across different categories, yet Korus sets itself apart by combining self-repositioning with monitoring capabilities. Traditional repositioning beds have been essential in healthcare. However, the challenge of repositioning patients persists at home, in hospitals, and long-term care facilities. Korus addresses several limitations of current market solutions by focusing on two areas: 1) Repositioning without human intervention; and 2) Repositioning in any of the four cardinal sleeping positions: face-up, prone, and both sides. Additionally, Korus does not resemble a medical bed; it looks like any other premium mattress. In the smart mattress category, Korus's closest competitors may offer monitoring and a similar form factor, but Korus uniquely provides automation and positioning in any position, overcoming the main limitation of current products in the market. The competitive environment includes:
- Self-Positioning Category: Korus is distinguished in this category by offering a self-repositioning system with autonomous capabilities, making it the only serious player in this niche. This suggests limited direct competition within the same niche. However, there are beds designed to turn patients, but these require patients to be attached to the bed and are primarily used in hospitals. Brands in this category include Rotorbed, Rotorprone, Prona-O2, and Freedom Bed.
- Smart Category: Brands such as ReST, Saatva Solaire, Sleep Number, Eight Sleep, and Tempur-Pedic offer smart mattresses. While they provide health monitoring and may adjust in the face-up position, they do not offer full repositioning capabilities.
- Luxury Category: Brands like Hästens, Kluft, Vi-Spring, Hypnos, and Duxiana are positioned as luxury offerings. They do not seem to offer smart or self-adjustable positioning features but may offer passive pressure management.
- Therapeutic Category: Companies such as Hillrom, Stryker, Invacare, Drive DeVilbiss, and ArjoHuntleigh are competitors offering medical-grade mattresses. Although these features may benefit patient care in face-up positioning, they typically lack the integration of smart technology and autonomous system capabilities that Korus provides.
- Adjustable Bed Category: Companies like Adjustable Bed Enterprises, Leggett & Platt, Serta Simmons, and Sleep Number offer beds that can be manually adjusted, often for comfort reasons, such as snoring but are also limited to the face-up position.
While new competitors may pose a threat to our market share, barriers such as stringent regulations, the high complexity of developing ML applications, strategic IP ownership, and capital requirements may deter new entrants. To maintain our lead, we will focus on continuous innovation, introducing new applications for Korus, employing an aggressive IP strategy, enhancing Korus's precision, and striving for excellence in product and service.
Traction
- We have completed Korus's beta prototype, integrating all the systems, including an app for Android and IOS and the first machine learning application with which we performed a successfully an IRB medical study for repositioning and position recognition at the Brigham and Women's Hospital with the following results: Publication: https://doi.org/10.1101/2024.01.02.23300653.
- We raised around $650K between 2020 and 2022 from non-dilutive sources including NIH ($503k) through an SBIR phase 1 and the Epilepsy Foundation ($125K) through the EF shark tank, demonstrating support from medical institutions and the Epilepsy community.
- We've submitted a proposal for an SBIR Phase 2 grant, targeting $3 million in additional non-dilutive funding, supported with a matching offer for another $3 million from a strategic investor.
- We have started a patient registry for the first clinical trial product.
- We have also assembled an outstanding technical team focused on machine learning and a session advisory board including Arthur Maxwell (Business and manufacturing), Eamonn Hobs (Regulatory and development), Steven Cohen (Corporate legal), Reg B. Wilcox III, PT, DPT, MS (Rehabilitation medicine), and Dr. Paul R. Albear, MD (Pressure injury prevention).
- Strategic Partnerships: We are in advanced discussions with manufacturing partners to ensure we can effectively meet anticipated demand and scale operations.
- Regulatory Progress: We are well underway to obtain the necessary certifications and quality standards for a consumer-grade release, which is essential for our go-to-market strategy.
Due Diligence Docs
Please note that access to the company's confidential materials is limited. Click this button to request access from the Company and its representatives.
Updates
No updates found .
Supporters
Help us find best new ideas to fund by telling us what you think. Your feedback goes straight to the team behind this project in private, so tell them what you really think.
Index Score
1
Score
0
Score
1
Likes0
Partners0
Pilots0
Follows-
This campaign has ended but you can still get involved.See options below.
Help us find best new ideas to fund by telling us what you think. Your feedback goes straight to the team behind this project in private, so tell them what you really think.


