Restoring Health and Youth: HF Healthcare: Real Solutions for Aging & Chronic Disease
by Clarence Teo

We help patients reclaim life from aging and chronic illness—visible recovery, renewed energy, and measurable results in weeks.
Singapore, Singapore Alternative Therapies LongevityAbout our project
The problem we solve: Problem We’re Solving Chronic diseases and aging are the leading global health burdens—cancer, diabetes, stroke, and dementia now account for over 70% of deaths worldwide. In Asia alone, more than 400 million people live with chronic illness, with healthcare costs rising unsustainably. Yet hospitals largely manage symptoms with drugs and procedures, while wellness resorts offer only lifestyle relief—neither addresses root causes. Patients face high costs, poor outcomes, and little hope for real recovery. HF Healthcare bridges this gap by delivering evidence-based regenerative medicine with resort-based recovery, offering a path to restore health, extend vitality, and reduce costs. With just $1M of our $10M plan, we are ready to activate our first center, already backed by teams and patients eager for solutions.
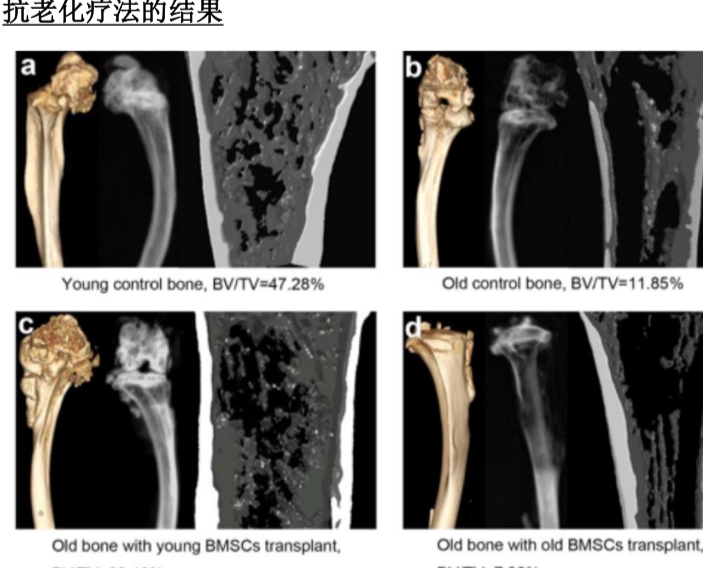
About our solution: At HF Healthcare, we help people restore vitality, reverse decline, and feel young again—often within just weeks. Unlike conventional hospitals that only manage symptoms, our unique therapies address the root causes of illness and aging. From easing knee and hip pain, to skin rejuvenation that looks 10 years younger, to helping patients with even Stage IV cancer achieve recovery, our methods combine the best of natural healing and breakthrough regenerative medicine. With stem cells, ozone, HBOT, IV nutrition, and more—all in one center—we give patients real hope, real results, and a chance to live longer, healthier lives.

Progress to date:
HF Healthcare is set to launch its first Wellness Center in Kuala Lumpur within 4 months of funding, backed by years of groundwork. With Singapore and Malaysia facing rapidly aging populations and soaring chronic disease rates, our model offers a breakthrough alternative to costly surgery and lifelong medication. Instead of high advertising spend, we have assembled a commission-based sales network of over 20 agents and team leaders—proven scalable in industries like life insurance—to drive immediate patient acquisition. Early traction includes interest from respected healthcare leaders, doctors, influencers, Bollywood networks, and European footballers. A prime location has already been secured, with renovation plans ready to create a world-class, trusted environment that inspires both patients and partners.
About Our Team

Creator: Clarence Teo
Location: Berat
Bio: Health Innovator & Visionary Clarence brings 20+ years of experience in integrative and restorative medicine, transforming his own severe health challenges—including heart disease, ulcerative colitis, and chronic fatigue—into a mission to redefine chronic disease and aging care. Through natural detoxification, nutrition, and regenerative protocols, he reversed conditions that conventional medicine could not—making him a living proof of his approach. Mentored by global pioneers including Charlotte Gerson, Dr. Johanna Budwig, and Dr. William Kelley (whose cancer protocol achieved >90% late-stage success rates, validated by Dr. Nicholas Gonzalez), Clarence built a rare foundation of clinical knowledge and practical application. Track Record: • Founded and managed health & wellness projects in Singapore, Malaysia, and China. • Guided patients with cancer and chronic illness toward measurable recovery. • Blends Western science with Eastern practices for practical, cost-effective solutions. Vision: To redefine healthcare through an ecosystem that merges nutritional therapies, detoxification, regenerative medicine, and anti-aging solutions into accessible, patient-centered care across Asia. .
Title: Founder & CEO
Advanced Degree(s): Functional Medicine University
About Team Members
GuiFang Teng
Co-Founder, China healthcare management certification
Biography: Patient-Care & Protocol Specialist
With extensive experience managing patients under Dr. Kelley’s treatment protocol in China, GuiFang brings deep practical expertise in integrative cancer and chronic illness care. She is dedicated to advancing natural healthcare solutions and ensuring clinical consistency in every center we establish.
Title: Co-Founder
Advanced Degree(s): China healthcare management certification
Greg King
Co-Founder, MBA
Biography: Global Healthcare & Technology Leader
Greg is a healthcare executive with 40+ years of international experience spanning Australia, New Zealand, South Africa, UK, USA, and Asia. He has led organizations across healthcare delivery, health IT, mergers & acquisitions, and consumer health innovation.
Key Achievements:
• Scaled a dual-listed health tech company (UK & Australia) to $500M+ market cap.
• Led M&A, integration, and governance for multiple healthcare firms in Asia.
• Pioneered China’s “triple-play healthcare” model (video, social, mobile) connecting patients and providers.
• Directed hospital systems, payer-based models, and multinational healthcare organizations globally.
Expertise:
Healthcare delivery, health IT, M&A, investor relations, consumer health, and preventive care. Greg’s leadership bridges cutting-edge healthcare with scalable business execution, ensuring HF Healthcare is built for global growth.
Title: Co-Founder
Advanced Degree(s): MBA
About Our Company

Health Factor (Asia Pacific) Pte Ltd
Location: 8 Temasek Boulevard, Suntec Tower Three
42-01
Singapore, 01 038988
SG
Founded: 2004
Product Stage: Ready
Employees: 1-2
Innovation Details
Intellectual Property Summary
HF Healthcare’s proprietary protocol combines natural pluripotent stem cells (nPSCs/VSELs) with exosomes in a 3-phase wellness model: body priming, regenerative therapy, and post-treatment support. Unlike conventional MSC injections, our approach delivers younger, more viable cells with superior integration and outcomes. This IP has been refined over years of practice, validated through patient results, and designed for scalable healing resorts.
Clinical Information
Clinical Efficacy (Summary)
Our protocol integrates elements with peer-reviewed support while we generate our own clinical evidence:
-
Endogenous pluripotent-like cells (VSEL/nPSC): Reviews describe rare, very small embryonic-like stem cells in adult tissues with multi-lineage potential; translational use remains early-stage and debated—supporting our need for controlled studies. PMCFrontiers
-
Exosome/EV support: Stem-cell–derived extracellular vesicles show anti-inflammatory and pro-regenerative effects across preclinical models and early clinical exploration. PMCBioMed CentralFrontiersNature
-
Hyperbaric oxygen (HBOT): Human studies show HBOT can mobilize circulating stem/progenitor cells and modulate cytokines. Physiology Journals+1PMC
-
Chelation (EDTA) in post-MI patients: The NIH-funded TACT trial reported reduced composite CV events, with stronger signals in diabetes—illustrating potential in specific cohorts. JAMA NetworkPubMed
-
Ozone-based modalities (incl. EBOO concepts): Evidence maps and mechanistic reviews suggest analgesic/anti-infective/anti-inflammatory effects; high-quality RCT data remain limited—another rationale for our trials. PMC+1Frontiers
-
High-dose Vitamin C: Robust preclinical oncology data and evolving clinical interest justify disciplined, indication-specific evaluation. PMC+1
Regulatory Status & Plan
-
United States: No FDA-approved exosome products; cell/exosome therapies generally regulated as HCT/Ps, biologics, or drugs (21 CFR Part 1271). We will not market in the U.S. without appropriate IND/IDE/biologics pathways. U.S. Food and Drug Administration+2U.S. Food and Drug Administration+2Federal Register
-
Current pathway: Initial IRB-approved pilot studies in Malaysia to assess safety, feasibility, and functional outcomes of our multi-phase protocol (priming + nPSC-aligned mobilization + EV support + aftercare). Primary endpoints: adverse events, validated pain/functional scores (e.g., WOMAC for knee OA), quality-of-life (EQ-5D), and lab biomarkers at 4, 12, and 24 weeks.
-
Data integrity: Prospective registration, independent monitoring, and publication plan.
How the Crowd Can Help
-
Fund pilots: US$1M to launch KL site and run two pilot cohorts (e.g., degenerative joint pain and metabolic syndrome), powering signal detection.
-
Enroll participants & providers: Community sign-ups for IRB studies; partner clinics for referrals and follow-up.
-
Regulatory acceleration: Budget for biostatistics, QA, GMP supply, and preparatory work for subsequent multi-center trials and, where applicable, U.S. IND discussions. U.S. Food and Drug Administration
Regulatory Status
Our innovation is gaining traction even before trials, with high-profile individuals already seeking nPSC-based therapies abroad—proof of unmet demand.
Regulatory status:
-
Exosome component delivered under Conditioned Medium (CM) safety frameworks.
-
nPSC (natural Pluripotent Stem Cell) technology under active patent filing.
Roadmap:
-
Near-term: IRB-approved pilot studies.
-
Mid-term: Expand regulatory submissions & clinical collaborations.
-
Long-term: Commercialize IP-protected nPSC + exosome protocols.
Unlike MSCs (marketed by cell count but limited in potency), nPSCs are naturally pluripotent and more regenerative. Adoption has been limited by commercialization challenges—not science.
Our protocol bridges this gap with a structured system (priming, exosome-guided differentiation, post-care) that ensures efficacy, scalability, and market viability.
How we will use the funds raised
Use of Funds ($1.5M Total):
- Medical Equipment & Inventory – $563,000 (37.5%)
(Saunas, ozone systems, bio-resonance, HBOT, colonics, microneedling, cryotherapy, injectables & peripherals) - Renovation & Furnishing – $700,000 (46.7%)
(Fit-out of 10,000 sq. ft. wellness center) - Rental & Utilities Deposit – $35,000 (2.3%)
(3.5 months deposit for facility lease) - Advertising, Promotion & Training – $100,000 (6.7%)
(Marketing campaigns & staff onboarding) - Contingency Fund – $102,000 (6.8%)
(Buffer for unforeseen costs & scale-up needs)
Thank You
I’ve spent years developing a wellness solution that gives people hope—without needing to rely only on drugs or surgery. Too many of my peers are already suffering from aging and chronic conditions, and I believe we can do better. With your support, we can open a Wellness Center in Kuala Lumpur that provides real, restorative care for those who need it most. Please vote for our project and help us bring relief, recovery, and renewed quality of life to many who are waiting for answers.
Updates
No updates found .
Supporters
Help us find best new ideas to fund by telling us what you think. Your feedback goes straight to the team behind this project in private, so tell them what you really think.
Index Score
1
Score
0
Score
1
Likes0
Partners0
Pilots0
Follows-
This campaign has ended but you can still get involved.See options below.
Help us find best new ideas to fund by telling us what you think. Your feedback goes straight to the team behind this project in private, so tell them what you really think.


