Ceeable: Cloud based eye exam
Web enabled technology to reduce blindness
Somerville, MA United States Medical Device Healthy Living BigData MedStartr Ventures challengeAbout our project

The problem we solve: The rapidly growing population of over 285 million visually-impaired patients go blind due to lack of access to easy and affordable screening.
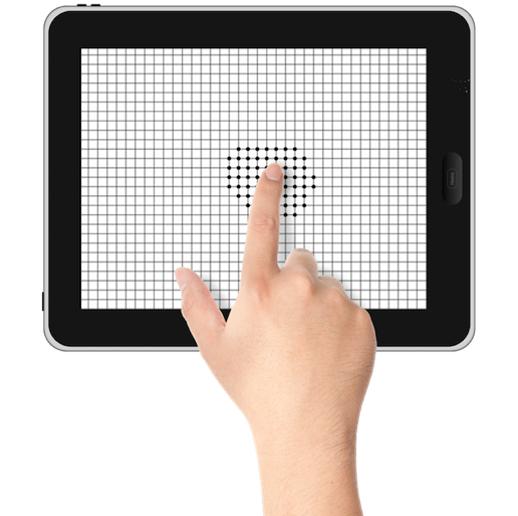
About our solution: The Ceeable visual field analyzer technology is a revolutionary visual field test that can detect, classify and monitor degenerative eye disease using only a tablet.

Progress to date:Ceeable Inc. was formed in 2014 to commercialize the mobile digital health Ceeable Visual Field Analyzer technology (CVFA). CVFA is the first of many products that will disrupt the traditional forms of patient ocular assessment, diagnosis and treatment. The technology has been employed in vision clinics in the US, Mexico, Russia and other developed and developing countries. The technology is based on a Caltech patented innovative software-based solution that runs on low cost commodity and ubiquitous touch-screen tablet and smartphone hardware. This approach serves as an alternative to the status-quo in the medical industry, which is typically dependent on expensive, bulky, and dedicated hardware products. The Ceeable products can be used in the clinic, remote settings or in the home by the individual patient. The Ceeable test has been used on over 3000 patients across the globe and has had a significant visual health impact on those with limited access to standard clinics.
About Our Team
Creator: Christopher Adams
Location: Massachusetts
Education: University of Massachusetts
Bio: Mr. Adams has founded and served as CEO of several companies including Diopter Corporation, and Mosaic Technologies. Mr. Adams was instrumental in raising venture capital, forming scientific and business advisory boards, recruiting and hiring key employees, and negotiating key corporate agreements and research collaborations. Diopter invented and developed a biological contact lens to treat corneal scarring. The technology has been used on over 25,000 patients.
Hospital Affiliation: Massachusetts Eye & Ear Hospital
Title: CEO
Advanced Degree(s): BA
About Our Company

Ceeable, Inc.
Location: 411A Highland Ave
Somerville, MA 02144
US
Founded: 2014
Website: http://ceeable.com
Twitter: @ceeable
Product Stage: In the Market
YTD Sales: Less than $250,000
Employees: 3-5
How We Help Patients
The company will offer CVFA in the form of intelligent tele-medical technology to home users. This is a natural progression for the technology as many people already have tablets and touch-screen devices in the home. This phase will add the ability of medical professionals to monitor their patients on a reoccurring basis (monthly, weekly, etc.) without the need to physically visit a medical facility. The patient’s home-based test results will be immediately accessible by their medical professional for evaluation and further treatment recommendations. This type of technology application would not be possible with existing large and complex perimetry systems.
How We Help Physicians
CVFA is an accurate, low cost, and fast visual field testing system that is accessible anywhere in the world, and the technology is applicable to a wide variety of markets and conditions. The ability to provide medical professionals with an independent second opinion (diagnosis) and progression monitoring capabilities will be an important tool for clinicians and caregivers.
How We Help Hospitals
Ceeable has developed a mobile digital health 3D Visual Field Analyzer (CVFA). CVFA is a comprehensive, non-invasive, web-based screening and dynamic classification system that can be easily used in clinical settings to detect early changes in visual function due to retinopathies such as glaucoma, macular degeneration, and optic neuropathy as well as progression monitoring over time. CVFA is an accurate, low cost, and fast visual field testing system that is accessible anywhere in the world, and the technology is applicable to a wide variety of markets and conditions. With the additional ability to provide medical professionals with an independent second opinion (diagnosis) and progression monitoring capabilities (planned), the market applicability for CVFA is expected to expand even more.
The Ceeable technology can be used in the clinic, remote settings or by the individual patient. The Ceeable test has been used on over 3000 patients across the globe. Over 285 million people in the world are visually impaired, with conditions such as Glaucoma and diabetic retinopathy.
Organization Benefit
For clinic or health facility the Ceeable technology will offer the ability to screen and test patients as they enter the eye clinic prior to consultation with hospital based clinicians. The Ceeable test will increase diagnostic and hospital efficiencies by enabling patients to get tested and screened sooner and will allow the clinician to access test data upon examination. Based on the results the clinic will be able to direct the patient to the appropriate next confirmation exam. Based on our analysis the system will offer work flow and cost efficiencies not available with the use of standard visual field testing instruments.
Challenge Mission
Market Size
Over 285 million people in the world are visually impaired, of whom 39 million are blind and 246 million have moderate to severe visual impairment (WHO, 2015). It is predicted that without extra interventions, these numbers will rise to 75 million blind and 200 million visually impaired by the year 2020 (WHO, 2014). The main causes of blindness are cataract (47.8%), glaucoma (12.3%), age related macular degeneration (8.7%), and diabetic retinopathy (4.8%) (WHO, 2010), with the latter being on the rise.Projected 3 Year Growth
Ceeable expects to achieve an annual run rate of over $100M within 5 years of product launch. At launch the gross margin will be 60% rising to greater than 90% by year five.How We Will Make Money
The company employs a “software as a service” business model (SAAS) where its software is centrally hosted and licensed for use by clients on an annual subscription basis.About our Competition
Automated perimetry systems are widely used to detect dysfunction in central and peripheral vision which may be caused by various medical conditions such as glaucoma, macular degeneration, stroke, brain tumors or other neurological deficits. The state of the art “gold standard” is the Humphrey Visual Field Analyzer. The product line is marketed by Carl Zeiss Meditec, Inc., and is the tool of choice for many ophthalmology and retinal clinics.CVFA provides several advantages over state-of-the-art conventional/automated perimetry systems such as the Humphrey Visual Field Analyzer: Cost, test time, 3D vs 2D, angular resolution, size and mobility.Innovation Details
Intellectual Property Summary
Invented at Caltech the patented visual filed analyzer (CVFA) is a comprehensive, non-invasive, web-based screening system that can be easily used in clinical settings to detect early changes in visual function due to retinopathies such as glaucoma, macular degeneration, and optic neuropathy as well as progression monitoring over time. The IP covers the use of an Amsler like grid coupled with variable contrast ratios to build a 3-Dmap of visual field defects based on a proprietary algorithm.
Patent Link
http://autonomy.caltech.edu/patents/US9122956.pdf
Clinical Information
The Ceeable technology is a clinically validated platform that has been tested on over 3000 patients. A recent study published in the British Journal of Ophthalmology titled “Early detection of glaucoma by means of a novel 3D computer-automated visual field test” demonstrated that the CVFA technology could detect the early onset of Glaucoma in several individuals before it was apparent using the Humphrey Visual Field Analyzer (Nazemi et al., 2007). The early onset detection of macular degeneration has also been reported in one of the studies (Nazemi et al., 2004). Further, another unrelated study recently demonstrated that CVFA could detect large-scale visual field defects in patients with ethambutol-induced optic neuropathy well before they were detectable by standard-automated perimetry (Kim et al., 2008). A study published in 2016 demonstrated the superiority of the Ceeable technology over microperimetry in determining small retinal defects (Milyutkina, et.al. 2016). Although the research on early detection and classification of visual field defects with CVFA is still in process, the initial results are very promising.
Regulatory Status
Based on an extensive regulatory assessment, the Ceeable CVFA is class I exempt from FDA regulations. The exemption covers the general area of visual field examination technologies. There is a requirement that the product be registered as a medical device with the FDA and adhere to GMP manufacturing procedures prior to marketing.
How we will use the funds raised
The company will use the funds raised to further refine the CVFA platform. This will be an ongoing refinement process and is accomplished by updating the single server location. The use of funds is segmented as follows: 10% on R&D and intellectual property development, 9% on G&A, 23% on engineering, such as platform expansion (Android, Windows), 58% on marketing and sales. The company has a current marketing effort in central and south America with north America starting in Q1 2017. The projected product gross margins are greater than 85% rising to 90% in year 3.
Thank You
Ceeable’s goal is to deploy the CVFA technology across the globe to reduce the incidence of blindness. For the first time a software based technology that runs on low cost commodity and ubiquitous touch-screen tablet and smartphone hardware can perform a visual field exam for retina and optic nerve disease with the same precision and accuracy as machines that are many times greater in cost and complexity.
Updates
No updates found .
Supporters
-
, Master’s Degree in Business Administration from Seton Hall University
10/06/2017 - Followed the project., Master’s Degree in Business Administration from Seton Hall University
10/06/2017 - Liked the project. , JD MPH
, JD MPH
11/20/2016 - Liked the project.
11/18/2016 - Liked the project.
11/18/2016 - Liked the project.
11/14/2016 - Liked the project. , BA
, BA
11/08/2016 - Liked the project.
11/08/2016 - Liked the project.
11/08/2016 - Liked the project. , MS
, MS
11/03/2016 - Liked the project.
Comments
-
erik Melling posted on 8th November, 2016
Phenomenal disruptive technology that will revolutionize the visual diagnostic world! No doubt that this will prevent scores of blindness, especially in countries who really need cost effective solutions.
Click here to LoginInstant Feedback
Help us find best new ideas to fund by telling us what you think. Your feedback goes straight to the team behind this project in private, so tell them what you really think.
35Medstartr
Index Score35
Interest
Score0
Adoption
Score9
Likes0
Partners0
Pilots1
Investors-
This campaign has ended but you can still get involved.See options below.
$20,000 pledged of $100,000 goal$20,000 Investor, Pilot & Parnter
interest to date.
Instant Feedback
Help us find best new ideas to fund by telling us what you think. Your feedback goes straight to the team behind this project in private, so tell them what you really think.


