NICGM real time blood glucose : Setting Diabetics Free
by Marc Rippen
Wearable truly noninvasive continuous glucose monitor and alert system that actually measures blood glucose levels real time (Not interstitial glucose like other CGM system currently available that all require sensor probes inserted into the skin or body)
Melbourne, FL United States Diabetes Equity Raise The Diabetes challenge NOLAHI Challenge - See AllAbout our project
The problem we solve: Over 20 million people in the United States have Diabetes, determined by CDC as of 2012. The number is growing. Most rely on intrusive blood sugar measurement methods requiring self measurement of blood from blood pricks multiple times every day (SMBG). If they are suffering from a low blood sugar attack they may not be able to be cognitive enough to do anything and could lapse into a coma. If they are living alone this could be deadly. Other continuous glucose monitors (CGM) are invasive requiring probes to be inserted into the skin that must be replaced and that can only measure interstitial glucose, which typically lags 10 minutes or more behind actual blood glucose measurements making it difficult for diabetics to make the right decisions real time to properly maintain their blood glucose levels real time.
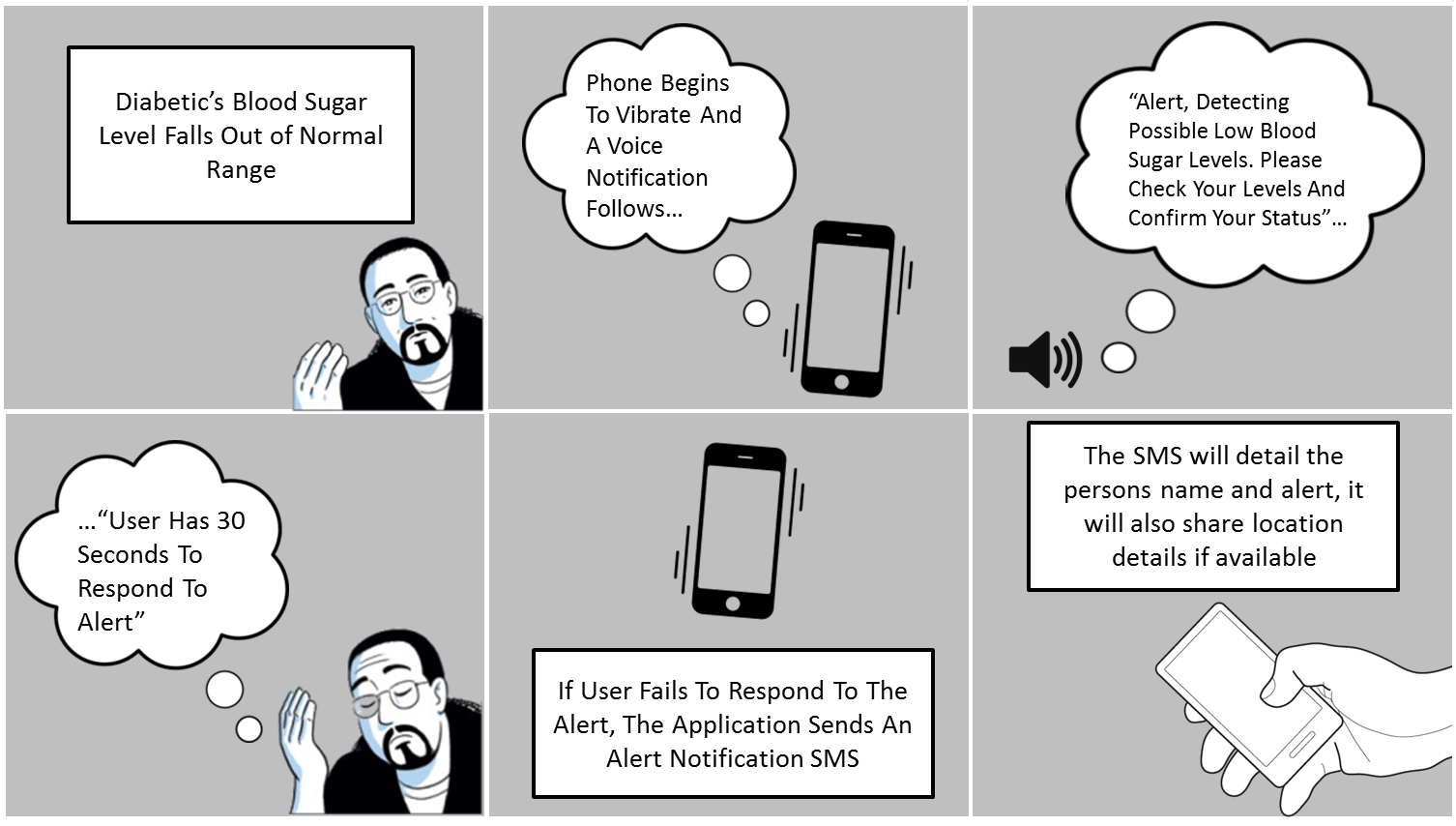
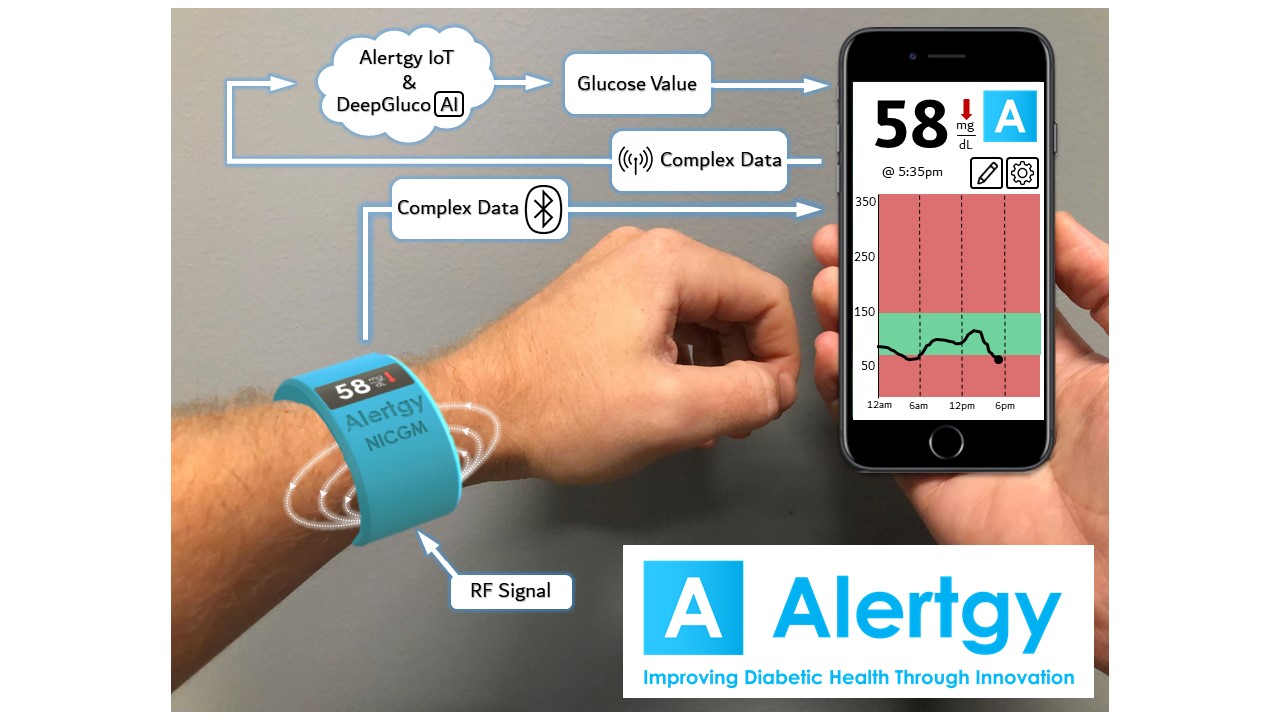
About our solution: Our device continually measures actual blood sugar levels using a non intrusive sensor embedded in a wrist band worn by the diabetic. The data collected by the sensor is used to calibrate the system through the Alertgy Proprietary IoT cloud driven AI analytics system and to continuously optimize its accuracy. If blood sugars start to go too high or too low the smartphone which is monitoring the wrist band using wireless technology notifies the user of the situation. If the user ignores or is unable to respond to their smart phone alert it will call the alert contacts that the user programs into his phone app. The phone will then text or call those contacts with an alert and a location where the phone is located so they can follow up to insure the user is OK. This allows the USER the freedom of not having to prick themselves, and insures that all measurements are being done so that the users physician can get an accurate assessment of that individuals blood sugar levels real time.

Progress to date:
We have a fully equipped systems development facility, A prototype system that has demonstrated equivalent performance to the hand held glucometers used to measure the blood from a users finger prick with a thumb based sensor. We also have 15 employees including a Chief Medical Officer MD (UF), PhD (in Biomedical Engineering Duke) and MPH (John Hopkins) with medical device public health experience. Check out our video on the technology page of our website www.alertgy.com.
About Our Team

Creator: Marc Rippen
Location: Florida
Education: Degrees in Analytical Chemistry, Microbiology
Bio: Entrepreneur, Scientist, Engineer and Leader. Established Alertgy to provide diabetics a real time non intrusive wrist band based blood sugar monitor enabled with a smart phone app to give people freedom from having to prick themselves to measure their blood sugar, to alert them and their loved ones if levels are too high or low and give them real time trend analysis.
Hospital Affiliation: Former Director of Engineering SRI International Adjunt Professor at Embry Riddle Aeronautical Univ
Title: CEO
Advanced Degree(s): MS
Twitter: @Alertgy
About Our Company

Alertgy Inc
Location: 5130 Commercial Drive
F
Melbourne, FL 32940
US
Founded: 2016
Website: http://www.alertgy.com
Product Stage: Prototype/MVP
YTD Sales: Working on it
Employees: 3-5
How We Help Patients
Alertgy will provide a means for diabetics not to have to prick themselves to know what their blood sugar level is. It will allow for a means to continuously moniter their blood sugar levels improving the ability for their doctors to monitor their continous history. It will allow for them to be alerted real time if their blood sugars are getting too high too low and if they are incapacitated by their blood sugar levels notify their loved ones doctors automatically. All this for less than what the blood strip finger prick glucomter system cost to use today.
How We Help Physicians
The Alertgy non invasive blood glucose monitering system will provide them with continous blood sugar data on their patients that can be sent / downloaded by cell phone at what ever frequency is required for proper diabetic patent monitering. Most people hate to prick themselves so by nonivassive real time monitoring doctorswill be able to get all the data they need, with no dependancy on the patient to do what they should do.
How We Help Hospitals
The Alertgy device allows for non invasive blood glucose monitering during ICU stays to improve recovery time from traumatic injuries oroperations. The data it generates can also be used to better monitor diabetics to better manage their disease and reduce the costs associated with poor management of diabetics. Reducing costs for amputation, vision and other long term health issues.
How We Help Partners
Alertgys non obtrusive blood glucose monitering will help employers and insurance providers with a means to empower their employees and their doctors with critical continous blood sugar data that can be used to improve the treatment of the employee and give the employee a means to optimize their behaviors based on instant feed back ability of the device. This will reduce sick time, health care costs as well as minimze incidents were employees pass out from low blood sugar at work and have to have emergency services rendered.
Challenge Mission
Market Size
Our Global Market is the 422 million people that suffer from diabetics worldwide1 (8.5% of the world population). 21.1 million are Americans that are diagnosed with diabetes. An additional 8.1 million Americans have diabetes but are not aware of it. So a total of 29 million Americans are affected2 (9.3%). Of the three types of diabetes (Type 1, Type 2, and gestational), type 2 accounts for over 90% of the cases of diabetes.
The increased prevalence of diabetes and obesity (a risk factor for type 2 diabetes) has increased dramatically from 1994 to 2014 and is expected to grow. There are 86 million Americans with pre-diabetes (1/3rd of the population) of which 15% to 30% will develop type 2 diabetes in five years. Diabetes is the seventh leading cause of death in the US and accounts for significant morbidity. People with diabetes are at risk for health complications including blindness, kidney failure, increased risk of heart attacks (1.8 times higher, death 1.7 times higher), strokes (hospitalization 1.5 times higher), and non-traumatic lower limb amputations5. In addition to the human costs, there are economic ones as well. The total medical costs and lost work and wages for people diagnosed with diabetes is $245 billion6. It has been estimated that the lifetime additional healthcare cost of one patient with diabetes is between $55,000 and $130,000.7 It is clear that diabetes has a significant impact on an individual’s health and our healthcare costs.
This data was obtained from 2 sources:
1) World Health Organization Website. “Diabetes Fact Sheet”. Updated November 2016. Obtained online 12/10/2016 at: http://www.who.int/mediacentre/factsheets/fs312/en/
2) CDC Website. “2014 National Diabetes Statistics Report” https://www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf
Projected 3 Year Growth
We are looking to do preclinical trials in early 2019, followed by clinical trials 3 months later, fda certification by end of 2020, market launch 2021.We expect to generate over 1 billion dollars in recurring revenue by end of the first year to market, with just 10% of US Market.
How We Will Make Money
We are the only truly non-invasive wearable technology that we know of. We bring to market a disruptive technology that addresses a major unmeet need in the mature diabetes industry: a totally non invasive manner. We will make money by providing our wristbands undera subscription agreement. Our cost per wristband is under $33.75 this include BOM, assembly, packaging and delivery. We will charge $100.00 a month which is less than supplies cost for glucometers. We will use a fully scalable 3 prong approach to market. Direct to consumer through the Internet, Business to business through retailers, and Business to Health Care providers through outreach and pilot projects.
About our Competition
Alertgy currently has no direct competitor in the market. 90% of the completion consists of the current manufacturers of prick and test strip glucometer systems. There are no truly non-invasive systems available to provide real time blood sugar levels and trends. Competitors such as Dexcom use sensors embedded under the patient’s skin or on the eye to achieve continuous monitoring which is extremely invasive. Optical and other skin chemistry methods are not practical for continuous monitoring and lack adequate selectivity and or sensitivity to meet FDA requirements. The skin chemistry based systems cause rashes on many patients since they require abrasion or fluid exchange through the skin to work. In addition all of these technologies are significantly more expensive and are not as easy to use.
Progress with Customers to date
We have raised 2.2 million in equity funding to date. We are presently at 50% of current 2 million dollar raise at a 25 million dollar pre valaution.
New Orleans and Our Company
If funded we will continue preclinical and clinical studies, develop fabricate optimize our wristband gen 2 product, start FDA approval Process, file for additional Patents conduct key hires, pursue strategic partnerships, and move out of our present incubator. We also would support research at local universitieds to look at other medical applications for out technology in other preventative medicine applications.
Innovation Details
Intellectual Property Summary
I am an inventor in several related patents, I have filed for a provisional utility patent for the core technology and demosntrated FTO, I will also be filing for other patents as we go, filing copyrights for software, have fied for several relevant trademarks, and will be treating our proprietary dielctric materials as a trade secret.
| Patents / Publication # | Year of Publication / Issued | Title | Citations |
|---|---|---|---|
| 2016/0005,552 | 2016 | High Permittivity Nanocomposites for Electronic Devices | 0 |
| 8,653,510 | 2014 | Enhanced E-field sensing using negative capacitance FET subthreshold slope enhancement | 0 |
| 8,321,759 | 2012 | Method and apparatus for error correction on a mobile device | 0 |
| 2012/0288,627 | 2012 | THREE-DIMENSIONAL ELECTROMAGNETIC METAMATERIALS AND METHODS OF MANUFACTURE | 3 |
| 2011/0147,723 | 2011 | ENHANCED E-FIELD SENSING USING NEGATIVE CAPACITANCE FET SUBTHRESHOLD SLOPE ENHANCEMENT | 1 |
Patent Link
http://www.patentbuddy.com/Inventor/RIPPEN-MARC/14278609
Clinical Information
Letter stamped August 28, 2002 from FDA on a similar device creates precedence for an FDA approval due non intrusive nature of the device:
Re: P990026/S008 Gluco Watch G2 Biographer Filed: September 19,2001 Amended: March 1, April 16, and, August 1, 2002
Dear Dr. Kersten:
The Center for Devices and Radiological Health (CDRH) of the Food and Drug Administration (FDA) has completed its review of your premarket approval application (PMA) supplement for the GlucoWatch G2 Biographer, which expands the indications for use to include children/adolescents (age 7 to 17) to the currently approved indications for use. This device is now indicated for the following:
• The GlucoWatch G2 Biographer is a glucose monitoring device indicated for detecting trends and tracking patterns in glucose levels in adults (age 18 and older) and children/adolescents (age 7 to 17) with diabetes. This device is intended for use by patients at home and in health care facilities. The device is for prescription use only.
•The GlucoWatch G2 Biographer is indicated for use as an adjunctive device to supplement, not replace, information obtained from standard home glucose monitoring devices.
•The Biographer is indicated for use in the detection and assessment of episodes of hyperglycemia and hypoglycemia, facilitating both acute and long-term therapy adjustments, which may minimize these excursions. Interpretation of Biographer results should be based on the trends and patterns seen with several sequential readings over time.
The PMA supplement is approved. You may begin commercial distribution of the device as modified in accordance with the "Conditions of Approval" (enclosed).
This device was not accurate enough for various reasons which we have addressed through the use of advanced dielectric materials and a the use of prorietary machine learning AI advanced IoT cloud based analytics to make the data more precise and accurate.
Regulatory Status
Once we complete the optimization of our device we will pursue pre-trials. We will then develop an ASIC to make the mass production of our device inexpensive, the first ASIC devices will then be submitted for FDA clinical trials with medical research institutions under the direction of our Cheif Medical Officer. Based on input from FDA consultan companies we will be considered to be a Class 1 device with a 510K approval process. We will start as a monitor and surveillance alert system to start much as DEXCOM and Abbott introduced their first CGM systems in the past.
How we will use the funds raised
We will use the money raised to take to reduce the exisitng clinical device to a smaller wristband product, key hires, pilot studies, file patents to protect our IP run pre clinical / clinical studies working with the FDA to gain necessary certifications, pursue strategic partnerships in manfuacturing, sales and distribution and moveout of the groundswell incubator where we are presently based.
Thank You
I started Alertgy after I saved my wife from lapsing into a diabetic coma. If I had not found her when I did and taken the necessary action she would probably be dead today. It really shook me up. I decided to do something about it. Alertgy was the name we came up with to Alert the g is for glucose and the y is because we need it. Given my dads recent death due to diabetic complications, my wifes transition from gestation diebetes to insulin dependent diabetes, I understood the nature of the problem more than most. To solve the probelm I founded Alertgy with a small team of innovators whom with I have collaborated on many challenging technical programs over the years for DARPA and DOD applications when I was the Director of Engineering at SRI. Now our focus was specifically to address the highly desired ability to measure blood sugar levels with an external wearable sensor device. The team has demonstrated taking technical laboratory concepts to proof of principal demonstrations, and scaling to global commercialization in several industries. We first undertook and completed an exhaustive review of the literature from the perspective of biology, analytical chemistry, physics and engineering, and have determined that it is possible to develop a non-intrusive blood sugar alert system, using existing technologies and manufacturing methods. To achieve an accurate measurement of blood sugar in a non-intrusive mode we realized the complexity of the problem from an analytical perspective. We have already demonstrated a wearale clinical device the size of a blood pressure cuff can acheive the necessary accuracy and precision required by the FDA to warrant the development of the next generation system. Both the current clinical and the next generation wristband platform are empowered by a smart phone application that links into a proprietary Alertgy cloud based IoT AI driven Analytics platform. We need your support to develop, build and test our gen 2 prototype, get FDA apoprovals and make it available to the 400 million diabetics world wide as soon as possible. Ironically in being a test subject for my clinical device I found I was exteremely hyper glycemic with a blood sugar reading ov 650 (anything over 150 mg/dl is bad). When the doctors did my A1C mine was 12.8 (anything over 7 is bad). I was one of 9 million diabetics in the US that didn't know he was diabetic and I wound up saving my own life. Thank you... Marc Rippen
Investor Info
Market Size
The total market opportunity is 20 million type one diabetics in the US and the other 400 million around the world. With 10% of the US Market Alertgy will be generating over 3 billion dollars of revenue on an annual basis.
Projected 3 Year Growth
In Three years after FDA approval Alertgy will be generating over 3 billion dollars in revenue with 10% of the US Market share, our earnings will allow us to rapidly address the 400 other diabetics world wide and be penetrating those markets as we move forward.
Revenue Model
Upon successful clinical validation of the accurate performance of our present clinical system which should be complete by early 2020, the company will enter into agreements with one or more strategic partners which have established sales and marketing resources. Our devices will be sold by those strategic partners “B to C” through the internet and “B to B” to health care organizations and drug store chains. Alertgy proposes a subscription pricing model at a monthly rate of $100 per unit to the end user. The monthly cost of providing that service is projected to be under $40, including the cost for manufacturing, packaging, and distributing a new wristband every month and providing an IoT cloud-based data analytics service. A ten percent market penetration of just the US market could generate $1 billion in recurring revenue to Alertgy and 2 billion to its partners. Alertgy plans to retain ownership and control of its proprietary sensor material fabrication and its cloud-based analytics software platform.
Competitors
Alertgy currently has no direct competitor in the market. Over 90% of the existing competition consists of manufacturers of finger prick and blood test strip glucometer systems. Continuous monitoring competitors such as Dexcom and Abbott use chemical reaction-based sensors embedded under the patient’s skin, Notably, the Abbott Libre system which features an invasive patch worn on the patient’s upper arm does not measure blood glucose levels. Instead the Libre examines interstitial (body) fluid. Glucose levels in that fluid typically lag actual blood glucose levels by approximately 20 minutes, making the measurement much less useful. Libre is generally not accepted as a substitute for blood glucose monitoring. Instead it is a warning system prompting the patient to test their blood glucose with a proven testing method. Dexcom’s devices require the implant of a sensor in the abdomen and also test interstitial fluid, not blood.
Traction
Alertgy raised 1.2 million in equity capital their seed round in 2018. They have raised another 1 million in equity capital with another 1 million to go in the present round of funding that just recently opened. Alertgy has developed a functional NICMG clinical product that provides continuous blood glucose measurements and trend analysis enabled by a smart phone application. The accuracy of the system has been validated by inhouse studies comparing Alertgy blood glucose readings to those produced by a leading “finger prick” blood glucose meter and lab benchtop systems. In the fall of 2019 Alertgy will sponsor an independent study at a major medical center to further confirm the accuracy of its blood glucose measuring system.
Starting with a large proof of principle system approximately the size of a home refrigerator in 2017, Alertgy has reduced the size of the NICMG to approximately that of a blood pressure cuff. That format is appropriate for hospital and clinical use. During the next 12-18 months the system will be further reduced to a wearable wristband size – much like a Fitbit or a smartwatch
Alertgy has accomplished the following other significant achievements to date:
- It was awarded a Space Coast EDC/NASA Tech Docking Grant in September 2017. Under this grant NASA scientists have validated the Alertgy dielectric spectroscopy rules engine and technology.
- Alertgy came in 2nd place at Biotechnology Florida Bio-Pitch Contest in Saint Petersburg Florida, competing with over 100 Health Technology start-up companies in the southeast.
- It came in second at the AMIA National Conference Pitch-IT event in Washington DC during November 2017, and was awarded a $7,500 grant.
- Alertgy came in first at the MEDMO2017 Medical Wearable Device Pitch Contest sponsored by Price Waterhouse Cooper at their world headquarters on Madison Ave in New York City during December 2017, with $25,000 prize.
- It was selected to participate in the first cohort of 10 companies chosen form hundreds of qualified applicants, to the Cleveland Clinic Plug and Play venture health technology accelerator program in February 2018. As part of this program Alertgy will be setting up preclinical trials and pilot studies with the Cleveland Clinic and other Cleveland based Health Care Systems during the Summer of 2018.
- Alertgy placed first at the NOLA Health Innovators Diabetes Challenge Pitch Finale in New Orleans during March 2018, as a result they were awarded a grand prize of $36,000.00. In addition to the cash prize Alertgy will be supported in setting up pre-clinical trials and pilot studies by Blue Cross Blue Shield, Tulane Health, Ochsner Health, and other organizations in New Orleans in the summer of 2018.
- Alertgy came in first place in SEMI executive conference Technology Showcase Pitch Event October 2018.
- Alertgy was recognized as one of top 30 fastest growing small businesses by the Silicon Reveiw Magazine
- Alertgy was recognized as one of the 10 ten Diabetic Care Providers for 2018 by Med-Tech Outlook Magazine
- Alerty was award $ 15,000 grant in FAU Tech Runway Pitch Event in 2019
- Alertgy came in first at NASA I-Tech Pitch Event in Orlando in September 2019 and is now in top 10 companies to pitch at NAS I-Tech Nation Event in October 2019 in New Mexico
Due Diligence Docs
Please note that access to the company's confidential materials is limited. Click this button to request access from the Company and its representatives.
Updates
No updates found .
Supporters
-

06/28/2021 - Interested in trying the project.
06/28/2021 - Followed the project. , test
, test
11/09/2018 - Interested in investing in the project. , MS
, MS
08/08/2018 - Interested in investing in the project.
03/27/2018 - Backed the project for $36000
03/25/2018 - Interested in investing in the project.
03/22/2018 - Followed the project.
03/22/2018 - Interested in a partnership with the project.
03/22/2018 - Interested in piloting the project. , MD
, MD
02/22/2018 - Liked the project.
01/30/2018 - Liked the project.
01/02/2018 - Liked the project.
11/19/2017 - Liked the project.
10/27/2017 - Liked the project.
10/25/2017 - Liked the project. Instant Feedback
Instant Feedback
Help us find best new ideas to fund by telling us what you think. Your feedback goes straight to the team behind this project in private, so tell them what you really think.
191Medstartr
Index Score146
Interest
Score9
Adoption
Score0
Likes1
Partners1
Pilots4
Investors-
This campaign has ended but you can still get involved.See options below.
$1,036,000 pledged of $1,000,000 goal$1,000,000 Investor, Pilot & Parnter
interest to date.
Instant Feedback
Help us find best new ideas to fund by telling us what you think. Your feedback goes straight to the team behind this project in private, so tell them what you really think.



